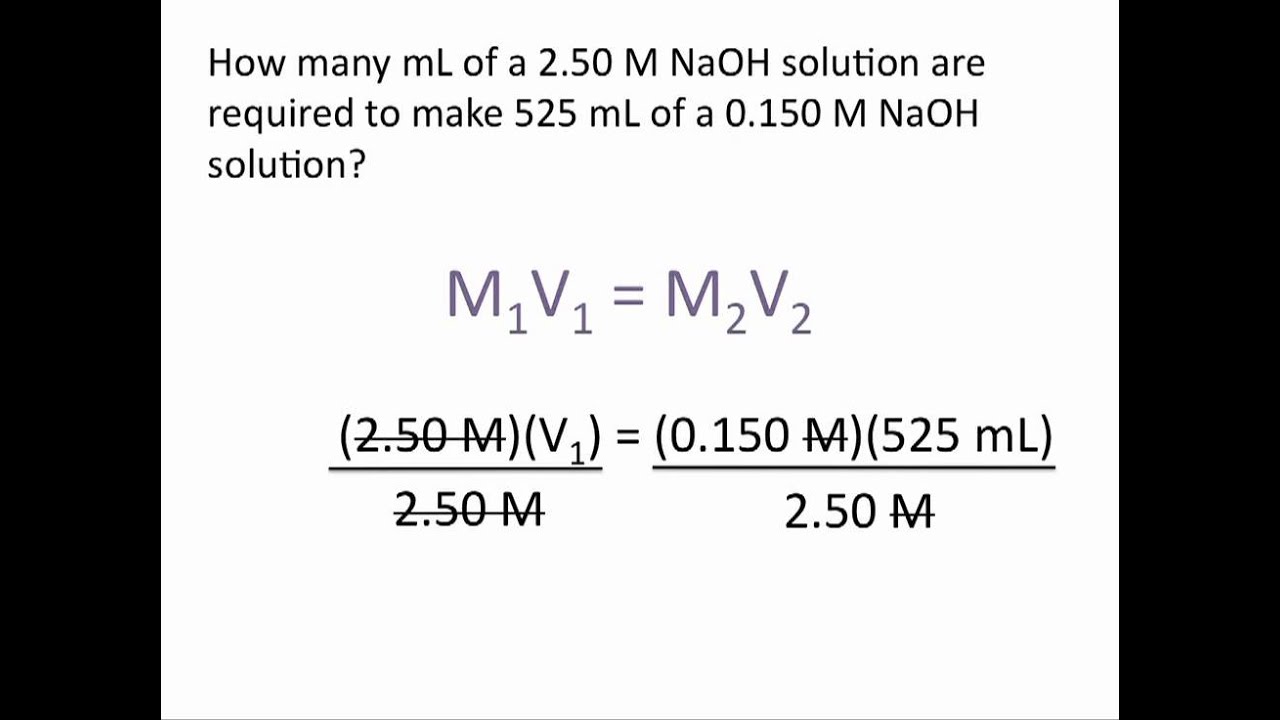
Formula For Dilution Factor Volume . Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. Df = v f v i = 10.0ml 0.1ml = 100. the dilution factor can be calculated using the following formula: formula to calculate dilution factor. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. calculate the new concentration or volume for a dilution or concentration of a solution. the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Dilution factor = v f / v i in this formula, v f represents the final. It may be expressed as the ratio of the volume of. dilution factor is the factor by which the stock solution is diluted.
from www.youtube.com
begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. Dilution factor = v f / v i in this formula, v f represents the final. dilution factor is the factor by which the stock solution is diluted. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. formula to calculate dilution factor. the dilution factor can be calculated using the following formula: Df = v f v i = 10.0ml 0.1ml = 100. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. It may be expressed as the ratio of the volume of. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution.
Dilution Problems Chemistry Tutorial YouTube
Formula For Dilution Factor Volume formula to calculate dilution factor. It may be expressed as the ratio of the volume of. the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. the dilution factor can be calculated using the following formula: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. calculate the new concentration or volume for a dilution or concentration of a solution. Df = v f v i = 10.0ml 0.1ml = 100. dilution factor is the factor by which the stock solution is diluted. formula to calculate dilution factor. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. Dilution factor = v f / v i in this formula, v f represents the final. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Formula For Dilution Factor Volume calculate the new concentration or volume for a dilution or concentration of a solution. formula to calculate dilution factor. dilution factor is the factor by which the stock solution is diluted. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. the dilution factor can be calculated using the following. Formula For Dilution Factor Volume.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Formula For Dilution Factor Volume It may be expressed as the ratio of the volume of. Df = v f v i = 10.0ml 0.1ml = 100. the dilution factor can be calculated using the following formula: formula to calculate dilution factor. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. Dilution factor = v f /. Formula For Dilution Factor Volume.
From lasopamail784.weebly.com
Calculate Dilution Factor lasopamail Formula For Dilution Factor Volume Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. the dilution factor can be calculated using the following formula: dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. the dilution factor (or dilution ratio) is the notation used to express how much of the. Formula For Dilution Factor Volume.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Formula For Dilution Factor Volume Dilution factor = v f / v i in this formula, v f represents the final. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. Df = v f v i = 10.0ml 0.1ml = 100. the dilution factor (or dilution ratio) is the notation used to express how much of the original. Formula For Dilution Factor Volume.
From printablezoneunglad.z13.web.core.windows.net
How To Calculate Dilution Concentrations Formula For Dilution Factor Volume the dilution factor can be calculated using the following formula: begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. formula to calculate dilution factor. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. Dilution factor = v f / v i in. Formula For Dilution Factor Volume.
From dxobhpmpa.blob.core.windows.net
Dilution Mass Per Volume at Thomas Bowling blog Formula For Dilution Factor Volume Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. Dilution factor = v f / v i in this formula, v f represents the final. the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. . Formula For Dilution Factor Volume.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Formula For Dilution Factor Volume Df = v f v i = 10.0ml 0.1ml = 100. formula to calculate dilution factor. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. calculate the new concentration or volume for a dilution or concentration of a solution. the dilution factor (or dilution ratio) is the notation used. Formula For Dilution Factor Volume.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Formula For Dilution Factor Volume the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Dilution factor = v f / v i in this formula, v f represents the final. formula to calculate dilution factor. Df = v f v i = 10.0ml 0.1ml =. Formula For Dilution Factor Volume.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen Formula For Dilution Factor Volume dilution factor is the factor by which the stock solution is diluted. formula to calculate dilution factor. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. calculate the new concentration or volume. Formula For Dilution Factor Volume.
From www.youtube.com
How to Calculate Dilution Factor YouTube Formula For Dilution Factor Volume formula to calculate dilution factor. dilution factor is the factor by which the stock solution is diluted. the dilution factor can be calculated using the following formula: It may be expressed as the ratio of the volume of. Dilution factor = v f / v i in this formula, v f represents the final. Suppose you diluted. Formula For Dilution Factor Volume.
From www.freepik.com
Premium Vector Dilution factor formula science vector illustration Formula For Dilution Factor Volume dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. dilution factor is the factor by which the stock solution is diluted. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. formula to calculate dilution factor. V f = aliquot volume. Formula For Dilution Factor Volume.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Formula For Dilution Factor Volume Df = v f v i = 10.0ml 0.1ml = 100. the dilution factor can be calculated using the following formula: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. formula to calculate dilution factor. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a. Formula For Dilution Factor Volume.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog Formula For Dilution Factor Volume the dilution factor can be calculated using the following formula: dilution factor is the factor by which the stock solution is diluted. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. formula to calculate dilution factor. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the. Formula For Dilution Factor Volume.
From exosmtljy.blob.core.windows.net
Dilution Cell Density at Bryan Owen blog Formula For Dilution Factor Volume Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. It may be expressed as the ratio of the volume of. dilution factor is the factor by which the stock solution is diluted. calculate the new concentration or volume for a dilution or concentration of a solution. Df = v f v i. Formula For Dilution Factor Volume.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Formula For Dilution Factor Volume Dilution factor = v f / v i in this formula, v f represents the final. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. the dilution factor can be calculated using the following. Formula For Dilution Factor Volume.
From www.pdfprof.com
how to calculate dilution factor Formula For Dilution Factor Volume dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. It may be expressed as the ratio of the volume of. calculate the new concentration or volume for a dilution or concentration of. Formula For Dilution Factor Volume.
From quizdemurrages.z21.web.core.windows.net
Calculate Dilution Percent Formula For Dilution Factor Volume dilution factor is the factor by which the stock solution is diluted. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. It may be expressed as the ratio of the volume of.. Formula For Dilution Factor Volume.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Formula For Dilution Factor Volume the dilution factor can be calculated using the following formula: dilution factor is the factor by which the stock solution is diluted. It may be expressed as the ratio of the volume of. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. Df = v f v i = 10.0ml. Formula For Dilution Factor Volume.
From paghis.weebly.com
Easy way to do dilutions paghis Formula For Dilution Factor Volume the dilution factor can be calculated using the following formula: formula to calculate dilution factor. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution factor = v f / v i in this formula, v f represents the final. calculate the new concentration or volume for a dilution or. Formula For Dilution Factor Volume.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Formula For Dilution Factor Volume the dilution factor can be calculated using the following formula: the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Df = v f v i = 10.0ml 0.1ml = 100. V f = aliquot volume + diluent volume = (0.1. Formula For Dilution Factor Volume.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Formula For Dilution Factor Volume Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. the dilution factor can be calculated using the following formula: It may be expressed as the ratio of the volume of. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. the dilution factor. Formula For Dilution Factor Volume.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Formula For Dilution Factor Volume Df = v f v i = 10.0ml 0.1ml = 100. the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. It may be expressed as the. Formula For Dilution Factor Volume.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Formula For Dilution Factor Volume V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. Dilution factor = v f / v i in this formula, v f represents the final. Df = v f v i = 10.0ml 0.1ml = 100.. Formula For Dilution Factor Volume.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Formula For Dilution Factor Volume V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. formula to calculate dilution factor. Dilution factor = v f / v i in this formula, v f represents the final. the dilution factor can be calculated using the following formula: It may be expressed as the ratio of the volume of.. Formula For Dilution Factor Volume.
From www.slideshare.net
Molarity and dilution Formula For Dilution Factor Volume V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. It may be expressed as the ratio of the volume of. Dilution factor = v f / v i in this formula, v f represents the final. formula to calculate dilution factor. the dilution factor (or dilution ratio) is the notation used. Formula For Dilution Factor Volume.
From ar.inspiredpencil.com
Dilution Equation Formula For Dilution Factor Volume It may be expressed as the ratio of the volume of. dilution factor is the factor by which the stock solution is diluted. Dilution factor = v f / v i in this formula, v f represents the final. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. calculate the. Formula For Dilution Factor Volume.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Formula For Dilution Factor Volume Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. Df = v f v i = 10.0ml 0.1ml = 100. dilution factor is the factor by which the stock solution is diluted. the dilution factor can be calculated using the following formula: begin by determining the initial volume (v1) of the. Formula For Dilution Factor Volume.
From www.pdfprof.com
how to calculate dilution factor Formula For Dilution Factor Volume V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. Df = v f v i = 10.0ml 0.1ml = 100. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. . Formula For Dilution Factor Volume.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Formula For Dilution Factor Volume begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. It may be expressed as the ratio of the volume. Formula For Dilution Factor Volume.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Formula For Dilution Factor Volume dilution factor is the factor by which the stock solution is diluted. calculate the new concentration or volume for a dilution or concentration of a solution. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. Df. Formula For Dilution Factor Volume.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Formula For Dilution Factor Volume dilution factor is the factor by which the stock solution is diluted. Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution factor = v f / v i in this formula, v f represents the final.. Formula For Dilution Factor Volume.
From www.thetechedvocate.org
How to Calculate the Dilution Factor The Tech Edvocate Formula For Dilution Factor Volume the dilution factor can be calculated using the following formula: Df = v f v i = 10.0ml 0.1ml = 100. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. dilution. Formula For Dilution Factor Volume.
From www.youtube.com
Dilution Calculation Practice YouTube Formula For Dilution Factor Volume dilution factor is the factor by which the stock solution is diluted. the dilution factor can be calculated using the following formula: calculate the new concentration or volume for a dilution or concentration of a solution. It may be expressed as the ratio of the volume of. V f = aliquot volume + diluent volume = (0.1. Formula For Dilution Factor Volume.
From www.slideshare.net
Molarity and dilution Formula For Dilution Factor Volume Suppose you diluted 2 liters of juice with 3 liters of water, calculate the dilution. begin by determining the initial volume (v1) of the concentrated solution and the final volume (v2) after dilution. dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. calculate the new concentration or volume for a. Formula For Dilution Factor Volume.
From www.medicine.mcgill.ca
Serial Dilutions Formula For Dilution Factor Volume the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total solution after dilution. Dilution factor = v f / v i in this formula, v f represents the final. dilution factor is the factor by which the stock solution is diluted. Suppose you diluted. Formula For Dilution Factor Volume.