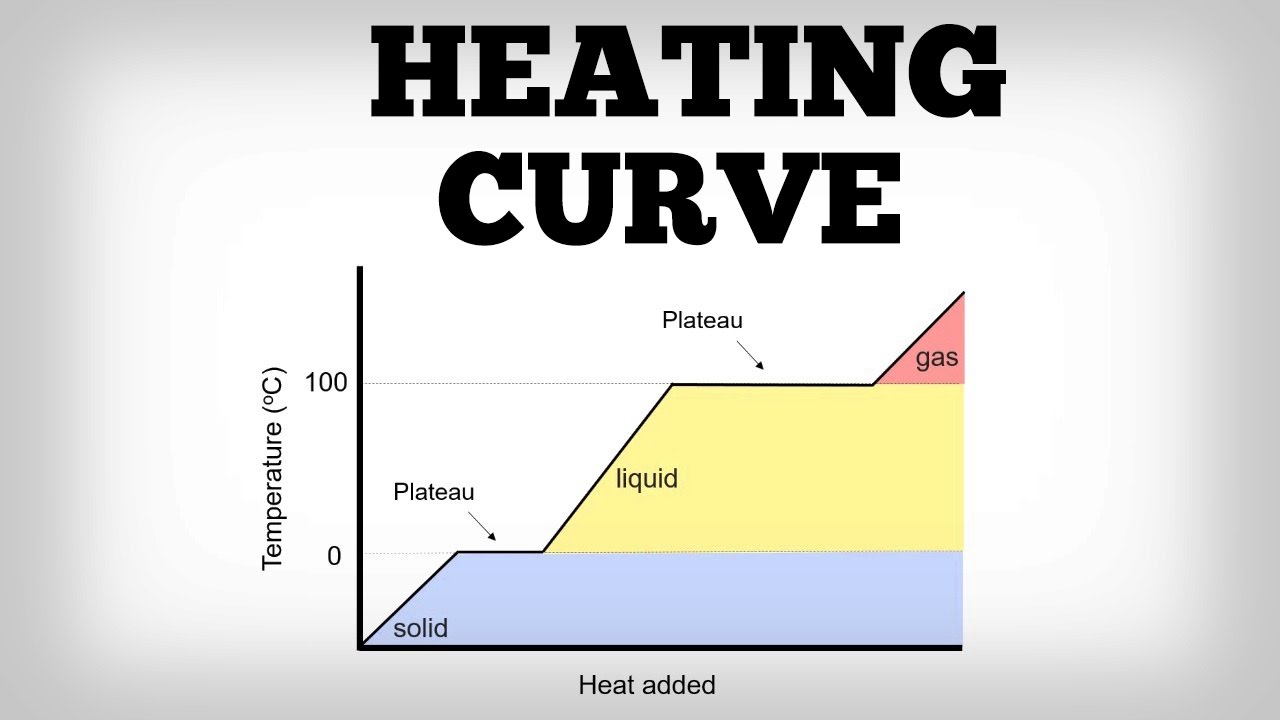
Heating And Cooling Curves Igcse . Cooling curves mirror heating curves, depicting the temperature changes as a substance cools down. The total time taken was 8 minutes. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. The plateaus on the curve. These show how the temperature of a substance increases with added energy. The gas <==> liquid <==> solid. A cooling curve is like a heating curve, but is the mirror image. The curve plateaus at phase changes (melting and. Heating and cooling curves are used to show how changes in. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. Like in the graph below: Plateaus on cooling curves indicate state. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. Starting from point ×, sketch on the. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be.
from lessonlibnurselings.z21.web.core.windows.net
The plateaus on the curve. Plateaus on cooling curves indicate state. These show how the temperature of a substance increases with added energy. Like in the graph below: Heating and cooling curves are used to show how changes in. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. The curve plateaus at phase changes (melting and. The gas <==> liquid <==> solid. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. Cooling curves mirror heating curves, depicting the temperature changes as a substance cools down.
Heating And Cooling Curve Chart
Heating And Cooling Curves Igcse The gas <==> liquid <==> solid. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. A cooling curve is like a heating curve, but is the mirror image. Like in the graph below: The gas <==> liquid <==> solid. These show how the temperature of a substance increases with added energy. Starting from point ×, sketch on the. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. The curve plateaus at phase changes (melting and. Heating and cooling curves are used to show how changes in. Plateaus on cooling curves indicate state. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. The plateaus on the curve. The total time taken was 8 minutes. Cooling curves mirror heating curves, depicting the temperature changes as a substance cools down.
From quizlet.com
Heating Curve for Water Diagram Quizlet Heating And Cooling Curves Igcse Heating and cooling curves are used to show how changes in. Starting from point ×, sketch on the. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. The curve plateaus at phase changes (melting and. The total time taken was 8 minutes. These show how the temperature. Heating And Cooling Curves Igcse.
From studyschoolburman.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curves Igcse The curve plateaus at phase changes (melting and. Like in the graph below: • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. Starting from point ×, sketch on the. A cooling curve is like a heating curve, but is. Heating And Cooling Curves Igcse.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.2 2 Pure Substance vs Mixture翰林国际教育 Heating And Cooling Curves Igcse Like in the graph below: Cooling curves mirror heating curves, depicting the temperature changes as a substance cools down. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse. Heating And Cooling Curves Igcse.
From lessonlibnurselings.z21.web.core.windows.net
Heating And Cooling Curve Chart Heating And Cooling Curves Igcse A cooling curve is like a heating curve, but is the mirror image. The gas <==> liquid <==> solid. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. These show how the temperature of a substance increases with added energy. Starting from point ×, sketch on the.. Heating And Cooling Curves Igcse.
From quizzlistreplevies.z13.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curves Igcse Starting from point ×, sketch on the. The curve plateaus at phase changes (melting and. Heating and cooling curves are used to show how changes in. Like in the graph below: The total time taken was 8 minutes. Plateaus on cooling curves indicate state. The gas <==> liquid <==> solid. A cooling curve is like a heating curve, but is. Heating And Cooling Curves Igcse.
From lessonlibnurselings.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curves Igcse Plateaus on cooling curves indicate state. The gas <==> liquid <==> solid. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. Cooling. Heating And Cooling Curves Igcse.
From www.savemyexams.com
Heating & Cooling Graphs AQA GCSE Physics Revision Notes 2018 Heating And Cooling Curves Igcse • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. The gas <==> liquid <==> solid. The plateaus on the curve. A cooling curve is like a heating curve, but is the mirror image. Cooling curves mirror heating curves, depicting. Heating And Cooling Curves Igcse.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Heating And Cooling Curves Igcse The plateaus on the curve. These show how the temperature of a substance increases with added energy. Like in the graph below: Heating and cooling curves are used to show how changes in. The curve plateaus at phase changes (melting and. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking. Heating And Cooling Curves Igcse.
From www.youtube.com
Latent Heat, Heating and Cooling Curves, Changes of State AQA GCSE Heating And Cooling Curves Igcse Like in the graph below: A cooling curve is like a heating curve, but is the mirror image. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. The gas <==> liquid <==> solid. A cooling curve represents the change. Heating And Cooling Curves Igcse.
From www.youtube.com
Heating and Cooling Curves IGCSE/ O level Chemistry / lec6 Chapter1 Heating And Cooling Curves Igcse Like in the graph below: • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. Starting from point ×, sketch on the. The. Heating And Cooling Curves Igcse.
From worksheetlistddt.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curves Igcse Like in the graph below: Heating and cooling curves are used to show how changes in. The gas <==> liquid <==> solid. Starting from point ×, sketch on the. The curve plateaus at phase changes (melting and. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. A cooling curve is like a heating. Heating And Cooling Curves Igcse.
From www.youtube.com
Heating and cooling curve video note by Mr Lee YouTube Heating And Cooling Curves Igcse The gas <==> liquid <==> solid. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. These show how the temperature of a substance increases with added energy. Plateaus on cooling curves indicate state. A cooling curve. Heating And Cooling Curves Igcse.
From worksheetlistddt.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curves Igcse The total time taken was 8 minutes. Starting from point ×, sketch on the. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. Cooling curves mirror heating curves, depicting the temperature changes as a substance cools down. The gas <==> liquid <==> solid. The plateaus on the curve. Heating and cooling curves. Heating And Cooling Curves Igcse.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating And Cooling Curves Igcse The curve plateaus at phase changes (melting and. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. Heating and cooling curves are used to show how changes in. The gas <==> liquid <==> solid. A cooling curve represents the. Heating And Cooling Curves Igcse.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating And Cooling Curves Igcse Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. The total time taken was 8 minutes. The curve plateaus at phase changes (melting and. The plateaus on the curve. The gas <==> liquid <==> solid. A. Heating And Cooling Curves Igcse.
From mmerevise.co.uk
Specific Latent Heat Questions and Revision MME Heating And Cooling Curves Igcse (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. The gas <==> liquid <==> solid. Cooling curves mirror heating curves, depicting the temperature changes as a substance cools down. The total time taken was 8 minutes. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves.. Heating And Cooling Curves Igcse.
From www.youtube.com
AP Video 10.6 Intro to HeatingCooling Curves & Calculations YouTube Heating And Cooling Curves Igcse The curve plateaus at phase changes (melting and. Like in the graph below: • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. A cooling curve is like a heating curve, but is the mirror image. Starting from point ×,. Heating And Cooling Curves Igcse.
From www.linstitute.net
CIE IGCSE Biology 复习笔记:1.1.2 States of Matter Heating And Cooling Curves Igcse The gas <==> liquid <==> solid. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. These show how the temperature of a substance increases with added energy. Changes of state in terms of kinetic particle theory, including the interpretation. Heating And Cooling Curves Igcse.
From mmerevise.co.uk
Specific Latent Heat Questions and Revision MME Heating And Cooling Curves Igcse The curve plateaus at phase changes (melting and. Starting from point ×, sketch on the. Like in the graph below: The plateaus on the curve. A cooling curve is like a heating curve, but is the mirror image. The total time taken was 8 minutes. Heating and cooling curves are used to show how changes in. Changes of state in. Heating And Cooling Curves Igcse.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating And Cooling Curves Igcse These show how the temperature of a substance increases with added energy. Plateaus on cooling curves indicate state. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. The curve plateaus at phase changes (melting and. (f) a sample of pure z was allowed to cool from 120. Heating And Cooling Curves Igcse.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources Heating And Cooling Curves Igcse Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. The plateaus on the curve. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. Like in the graph below: Plateaus on cooling curves indicate state. A cooling curve represents the change in temperature and phase transitions. Heating And Cooling Curves Igcse.
From worksheetlistddt.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curves Igcse A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. Plateaus on cooling curves indicate state. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. (f) a sample of pure z was allowed to cool from 120 °c to 20. Heating And Cooling Curves Igcse.
From getrevising.co.uk
States of Matter Revision Cards in IGCSE Chemistry Heating And Cooling Curves Igcse (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. The total time taken was 8 minutes. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. Like in the graph below: The curve. Heating And Cooling Curves Igcse.
From www.docsity.com
Heating and Cooling Curves (The Basics) Slides Applied Thermodynamics Heating And Cooling Curves Igcse The gas <==> liquid <==> solid. A cooling curve is like a heating curve, but is the mirror image. The total time taken was 8 minutes. Starting from point ×, sketch on the. Like in the graph below: Heating and cooling curves are used to show how changes in. The plateaus on the curve. Changes of state in terms of. Heating And Cooling Curves Igcse.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Heating And Cooling Curves Igcse Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. Heating and cooling curves are used to show how changes in. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would be. These show. Heating And Cooling Curves Igcse.
From lessonschoolimbrowning.z14.web.core.windows.net
Heating And Cooling Curves Worksheet Heating And Cooling Curves Igcse Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. The gas <==> liquid <==> solid. Heating and cooling curves are used to show how changes in. The curve plateaus at phase changes (melting and. Like in the graph below: A cooling curve represents the change in temperature and phase transitions that a. Heating And Cooling Curves Igcse.
From www.youtube.com
How to read and interpret heatingcooling curve YouTube Heating And Cooling Curves Igcse Like in the graph below: The total time taken was 8 minutes. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. A cooling curve is like a heating curve, but is the mirror image. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking. Heating And Cooling Curves Igcse.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating And Cooling Curves Igcse These show how the temperature of a substance increases with added energy. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. Starting from point ×, sketch on the. The curve plateaus at phase changes (melting and. Cooling curves mirror heating curves, depicting the temperature changes as a substance cools down. The gas. Heating And Cooling Curves Igcse.
From www.savemyexams.com
Investigating Cooling Curves for Stearic Acid Oxford AQA IGCSE Heating And Cooling Curves Igcse Cooling curves mirror heating curves, depicting the temperature changes as a substance cools down. The curve plateaus at phase changes (melting and. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. Plateaus on cooling curves indicate state. Starting from point ×, sketch on the. The gas <==> liquid <==> solid. These show. Heating And Cooling Curves Igcse.
From www.youtube.com
Heating Curves Cooling Curves in Physics GCSC(2019 IGCSE(2019 Heating And Cooling Curves Igcse A cooling curve is like a heating curve, but is the mirror image. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. The total time taken was 8 minutes. Heating and cooling curves are used to show how changes in. These show how the temperature of a substance increases with added energy. Plateaus. Heating And Cooling Curves Igcse.
From studylibsmith.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curves Igcse Like in the graph below: The gas <==> liquid <==> solid. The plateaus on the curve. Starting from point ×, sketch on the. A cooling curve is like a heating curve, but is the mirror image. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. The curve. Heating And Cooling Curves Igcse.
From coolingchiwayake.blogspot.com
Cooling The Cooling Curve Heating And Cooling Curves Igcse A cooling curve is like a heating curve, but is the mirror image. The curve plateaus at phase changes (melting and. Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. (f) a sample of pure z was allowed to cool from 120 °c to 20 °c. The total time taken was 8. Heating And Cooling Curves Igcse.
From www.youtube.com
IGCSE Chemistry p4. Heating curve..... YouTube Heating And Cooling Curves Igcse Changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. A cooling curve is like a heating curve, but is the mirror image. A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. Plateaus on cooling curves indicate state. The gas. Heating And Cooling Curves Igcse.
From www.pinterest.com
Heating and Cooling Curve Properties of matter, theory Heating And Cooling Curves Igcse A cooling curve represents the change in temperature and phase transitions that a substance undergoes as heat is taking out from it. Starting from point ×, sketch on the. Like in the graph below: A cooling curve is like a heating curve, but is the mirror image. These show how the temperature of a substance increases with added energy. The. Heating And Cooling Curves Igcse.
From lessonlibsertularia.z22.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curves Igcse The plateaus on the curve. These show how the temperature of a substance increases with added energy. Cooling curves mirror heating curves, depicting the temperature changes as a substance cools down. • these changes in state can be shown on a graph which is called a heating curve • cooling down a gas has the reverse effect and this would. Heating And Cooling Curves Igcse.