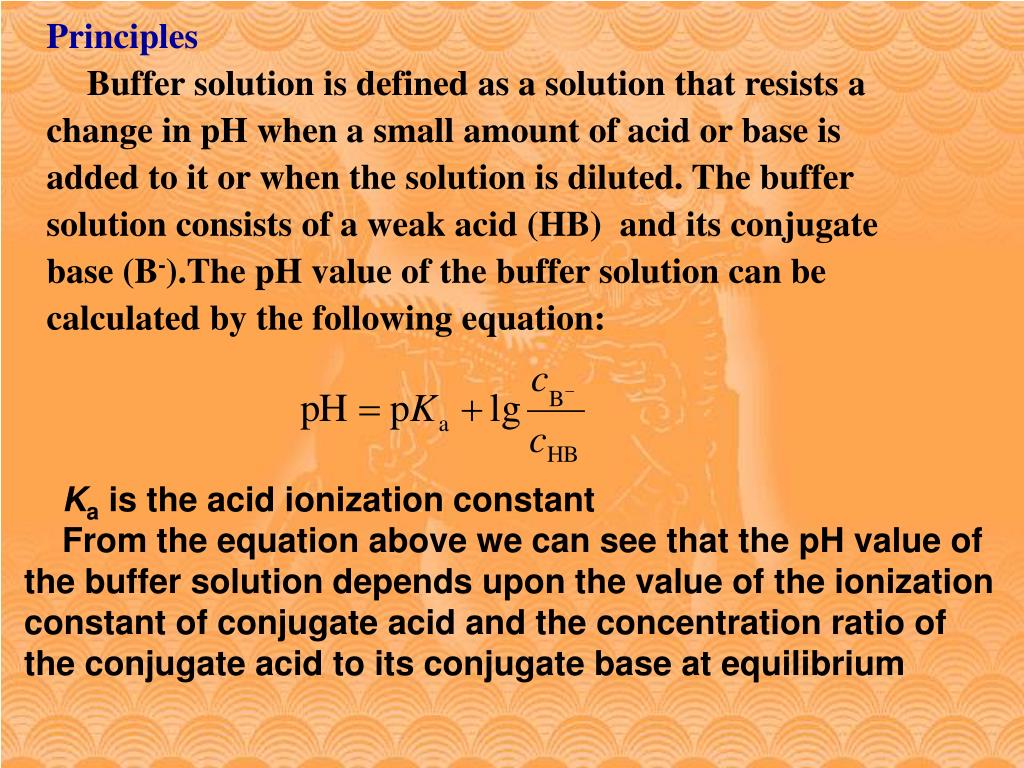
Lab Preparation Of Buffer Solutions . The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Find examples, equations, and references for weak. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. Learn what a buffer solution is, how to make and calculate its ph, and how it works. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. Øto learn how to prepare buffers. Øto understand the behaviour of buffers solutions.
from www.slideserve.com
Learn what a buffer solution is, how to make and calculate its ph, and how it works. Øto understand the behaviour of buffers solutions. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Øto learn how to prepare buffers. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Find examples, equations, and references for weak.
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint
Lab Preparation Of Buffer Solutions Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Øto learn how to prepare buffers. Øto understand the behaviour of buffers solutions. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. Learn what a buffer solution is, how to make and calculate its ph, and how it works. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Find examples, equations, and references for weak.
From www.slideserve.com
PPT Preparation of Buffers 1 PowerPoint Presentation, free download Lab Preparation Of Buffer Solutions Øto learn how to prepare buffers. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. Find examples, equations, and references for weak. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant. Lab Preparation Of Buffer Solutions.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog Lab Preparation Of Buffer Solutions Øto learn how to prepare buffers. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. See examples of acidic and basic buffers, such as acetic acid and sodium acetate.. Lab Preparation Of Buffer Solutions.
From www.scribd.com
5 Preparation of Buffer Solutions by Different Laboratory Ways PDF Lab Preparation Of Buffer Solutions Find examples, equations, and references for weak. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. Øto learn how to prepare buffers. The preparation of buffer. Lab Preparation Of Buffer Solutions.
From www.studocu.com
Bufferpreparation Buffer Solution Buffer Preparation (Shi Lab) 1 M Lab Preparation Of Buffer Solutions See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. Øto learn how to prepare buffers. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and. Lab Preparation Of Buffer Solutions.
From studylib.net
Preparation and Properties of Buffer Solutions Lab Preparation Of Buffer Solutions See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Øto learn how to prepare buffers. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Learn what a buffer solution is, how to make and calculate its ph, and how it works. Øto understand the behaviour of buffers solutions. This. Lab Preparation Of Buffer Solutions.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Lab Preparation Of Buffer Solutions Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Find examples, equations, and references for weak. Øto understand the behaviour of buffers solutions. Learn what a buffer solution is, how to make and calculate its. Lab Preparation Of Buffer Solutions.
From www.chegg.com
Solved Preparation of Buffer Solutions 0 EXPERIMENT 1 Lab Preparation Of Buffer Solutions This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Learn what a buffer solution is, how to make and calculate its ph, and how it works. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. The preparation of buffer solutions involves. Lab Preparation Of Buffer Solutions.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Lab Preparation Of Buffer Solutions Find examples, equations, and references for weak. Øto learn how to prepare buffers. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. Øto understand the behaviour of buffers solutions. Learn what a buffer solution is, how to make and calculate its ph, and how it works. The preparation of. Lab Preparation Of Buffer Solutions.
From www.chegg.com
Solved Preparation of Buffer Solutions 0 EXPERIMENT 2 Lab Preparation Of Buffer Solutions Øto understand the behaviour of buffers solutions. Learn what a buffer solution is, how to make and calculate its ph, and how it works. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Accurate preparation and correct selection of buffers. Lab Preparation Of Buffer Solutions.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Lab Preparation Of Buffer Solutions See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Find examples, equations, and references for weak. Learn what a buffer solution is, how to make and calculate its ph, and how it works. Øto understand the behaviour of buffers solutions. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account.. Lab Preparation Of Buffer Solutions.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Lab Preparation Of Buffer Solutions Learn what a buffer solution is, how to make and calculate its ph, and how it works. Øto understand the behaviour of buffers solutions. Find examples, equations, and references for weak. Øto learn how to prepare buffers. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. This lab experiment. Lab Preparation Of Buffer Solutions.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points Lab Preparation Of Buffer Solutions This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Learn what a buffer solution is, how to make and calculate its ph, and how it works. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Accurate preparation and correct selection. Lab Preparation Of Buffer Solutions.
From studylib.net
Buffer Solutions Lab Preparation Of Buffer Solutions Øto understand the behaviour of buffers solutions. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. Øto learn how to prepare buffers. Learn what a buffer solution is, how to make and calculate its ph, and how it works. This lab experiment involves measuring the ph of salt solutions, calculating the acid. Lab Preparation Of Buffer Solutions.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Lab Preparation Of Buffer Solutions Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. The preparation of buffer solutions involves. Lab Preparation Of Buffer Solutions.
From www.slideserve.com
PPT BUFFER SOLUTION PowerPoint Presentation, free download ID6580038 Lab Preparation Of Buffer Solutions This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. Find examples, equations, and references for weak. Øto learn how to prepare buffers. See examples of acidic. Lab Preparation Of Buffer Solutions.
From chemistrypubs.com
Buffer Solution Definition, Examples, Mechanisms, Preparations Lab Preparation Of Buffer Solutions Øto understand the behaviour of buffers solutions. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Learn what a buffer solution is, how to make and calculate its ph, and how it works. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. Øto learn how to. Lab Preparation Of Buffer Solutions.
From www.chegg.com
Preparation of Buffer Solutions EXPERIMENT 2 Lab Preparation Of Buffer Solutions Øto understand the behaviour of buffers solutions. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Learn what a buffer solution is, how to make and calculate its ph, and how it works. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Accurate preparation and correct selection of buffers. Lab Preparation Of Buffer Solutions.
From www.chegg.com
Solved Calculating the pH of a Buffer Solution To prepare a Lab Preparation Of Buffer Solutions Find examples, equations, and references for weak. Øto learn how to prepare buffers. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. This lab experiment involves measuring the ph of salt solutions, calculating the. Lab Preparation Of Buffer Solutions.
From www.youtube.com
Preparation and Analysis of Buffers Intro & Theory YouTube Lab Preparation Of Buffer Solutions See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Find examples, equations, and references for weak. Øto learn how to prepare buffers. Learn what a buffer solution is, how to make and calculate its ph, and how it works. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of. Lab Preparation Of Buffer Solutions.
From vocal.media
Preparation of Buffers Solution FYI Lab Preparation Of Buffer Solutions This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Øto learn how to prepare buffers. Find examples, equations, and references for weak. Øto understand the behaviour of buffers solutions. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Learn the properties. Lab Preparation Of Buffer Solutions.
From www.researchgate.net
1. Buffer solutions preparation method Download Table Lab Preparation Of Buffer Solutions Øto understand the behaviour of buffers solutions. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Learn what a buffer solution is, how to make and calculate its ph, and how it works. Find examples, equations, and references for weak. Learn the properties and factors of buffer solutions by preparing and measuring different buffer. Lab Preparation Of Buffer Solutions.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Lab Preparation Of Buffer Solutions Øto learn how to prepare buffers. Øto understand the behaviour of buffers solutions. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Find examples, equations, and references for weak. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. Learn the properties and factors of buffer solutions. Lab Preparation Of Buffer Solutions.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Lab Preparation Of Buffer Solutions This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Find examples, equations, and references for weak. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Learn what a buffer solution is, how to make and calculate its ph, and how. Lab Preparation Of Buffer Solutions.
From slidetodoc.com
EXPERIMENT 5 Preparation and Properties of Buffer Solution Lab Preparation Of Buffer Solutions Øto understand the behaviour of buffers solutions. Find examples, equations, and references for weak. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Learn what a buffer solution is, how to make. Lab Preparation Of Buffer Solutions.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Lab Preparation Of Buffer Solutions Øto understand the behaviour of buffers solutions. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Accurate preparation and correct. Lab Preparation Of Buffer Solutions.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Lab Preparation Of Buffer Solutions This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Øto understand the behaviour of buffers solutions. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from. Lab Preparation Of Buffer Solutions.
From www.chegg.com
Preparation of Buffer Solutions PRELAB QUESTIONS 1. Lab Preparation Of Buffer Solutions Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. Learn what a buffer solution is, how to make and calculate its ph, and how it works. Øto understand the behaviour of buffers solutions. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. See. Lab Preparation Of Buffer Solutions.
From www.studocu.com
Lab report 2 CHEM271 EXPERIMENT 2 BUFFERS AND BIOCHEMICAL ANALYSIS Lab Preparation Of Buffer Solutions Øto understand the behaviour of buffers solutions. Find examples, equations, and references for weak. Øto learn how to prepare buffers. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Learn the properties and factors of buffer solutions by. Lab Preparation Of Buffer Solutions.
From www.studocu.com
05 Buffers Procedure Fall22 CHM 116 LAB PROCEDURE Preparation of Lab Preparation Of Buffer Solutions Øto understand the behaviour of buffers solutions. Øto learn how to prepare buffers. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Accurate preparation and correct selection of buffers is essential to obtain reproducible and consistent results in capillary. Learn the properties and factors of buffer. Lab Preparation Of Buffer Solutions.
From www.studocu.com
Preparation of Buffer Solutions and Buffering Capacity Basanta Lab Preparation Of Buffer Solutions Find examples, equations, and references for weak. Øto understand the behaviour of buffers solutions. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Learn what a buffer solution is, how to make and calculate its ph, and how it works. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation. Lab Preparation Of Buffer Solutions.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation ID2841454 Lab Preparation Of Buffer Solutions Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Øto understand the behaviour of buffers solutions. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Find examples, equations, and. Lab Preparation Of Buffer Solutions.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Lab Preparation Of Buffer Solutions This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Øto learn how to prepare buffers. Øto understand the behaviour of buffers solutions. Find examples, equations, and references for weak. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic. Lab Preparation Of Buffer Solutions.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Lab Preparation Of Buffer Solutions The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Øto understand the behaviour of buffers solutions. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions. Lab Preparation Of Buffer Solutions.
From studylib.net
Experiment 7 Preparation of a Buffer Lab Preparation Of Buffer Solutions See examples of acidic and basic buffers, such as acetic acid and sodium acetate. Øto learn how to prepare buffers. Learn the properties and factors of buffer solutions by preparing and measuring different buffer solutions from acetic acid and sodium. Learn what a buffer solution is, how to make and calculate its ph, and how it works. Find examples, equations,. Lab Preparation Of Buffer Solutions.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Lab Preparation Of Buffer Solutions Øto learn how to prepare buffers. This lab experiment involves measuring the ph of salt solutions, calculating the acid dissociation constant of a weak acid, and designing a buffer. Øto understand the behaviour of buffers solutions. The preparation of buffer solutions involves a comprehensive approach that takes various factors into account. Learn the properties and factors of buffer solutions by. Lab Preparation Of Buffer Solutions.