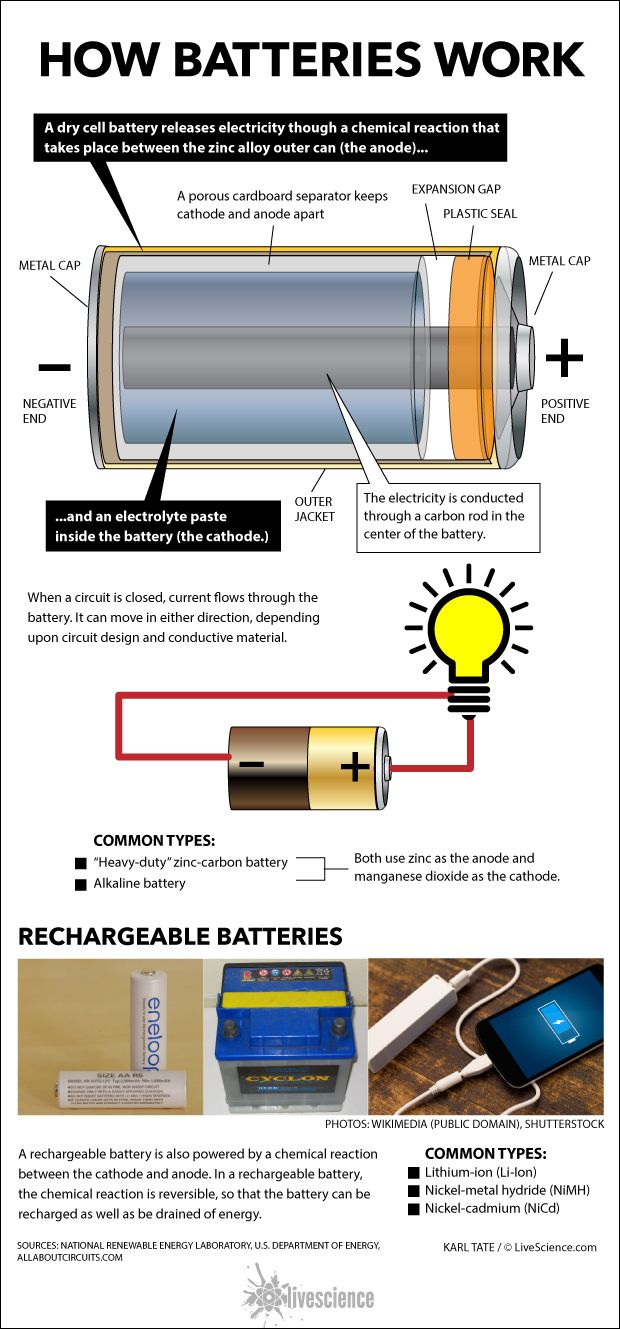
How Flow Battery Works . During discharge, electrons liberated by reactions on one side travel to the other side. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. They are rechargeable batteries that separate the energy storage. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. The ion exchange that occurs between the cathode and anode generates electricity. Flow batteries stand out from conventional batteries with their distinct operation and structure.
from www.livescience.com
Flow batteries stand out from conventional batteries with their distinct operation and structure. They are rechargeable batteries that separate the energy storage. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; During discharge, electrons liberated by reactions on one side travel to the other side. The ion exchange that occurs between the cathode and anode generates electricity. In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes.
How Do Batteries Work? Live Science
How Flow Battery Works During discharge, electrons liberated by reactions on one side travel to the other side. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; During discharge, electrons liberated by reactions on one side travel to the other side. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. They are rechargeable batteries that separate the energy storage. Flow batteries stand out from conventional batteries with their distinct operation and structure. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. The ion exchange that occurs between the cathode and anode generates electricity. In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane.
From diyguru.org
Redox Flow Battery Vs LiIon Working Principle Explained How Flow Battery Works The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; Flow batteries stand out from conventional batteries with their distinct operation and structure. During discharge, electrons liberated by reactions on one side travel to the other. How Flow Battery Works.
From techxplore.com
Scientists develop a better redox flow battery How Flow Battery Works In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. They are rechargeable batteries that separate the energy storage. A flow battery is a fully rechargeable electrical energy storage device where fluids containing. How Flow Battery Works.
From www.science.org
New generation of ‘flow batteries' could eventually sustain a grid How Flow Battery Works They are rechargeable batteries that separate the energy storage. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. During discharge, electrons liberated by reactions on one side travel to the other side. The. How Flow Battery Works.
From blog.upsbatterycenter.com
Wonder How a Flow Battery Works? News about Energy Storage, Batteries How Flow Battery Works The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. The ion exchange that occurs between the cathode and anode generates electricity. In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. They are rechargeable batteries that separate. How Flow Battery Works.
From www.livescience.com
How Do Batteries Work? Live Science How Flow Battery Works In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. They are rechargeable batteries that separate the energy storage. The flow battery is a form of battery in which electrolyte containing one or. How Flow Battery Works.
From whatswatt.com.au
Go with the flow What are flow batteries, and how do they work? What How Flow Battery Works During discharge, electrons liberated by reactions on one side travel to the other side. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. The ion exchange that occurs. How Flow Battery Works.
From www.youtube.com
Flow Battery Basics, Part 1 What They Are, How They Work, & Where They How Flow Battery Works They are rechargeable batteries that separate the energy storage. During discharge, electrons liberated by reactions on one side travel to the other side. The ion exchange that occurs between the cathode and anode generates electricity. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; Flow batteries stand out from conventional batteries with their distinct operation and structure.. How Flow Battery Works.
From www.slideserve.com
PPT Flow batteries for energy storage PowerPoint Presentation, free How Flow Battery Works The ion exchange that occurs between the cathode and anode generates electricity. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; Flow batteries stand out from conventional batteries with their distinct operation and structure. A flow battery is a fully. How Flow Battery Works.
From www.dreamstime.com
How a Lithium Ion Battery Works, 3d Elements Section. Battery Charging How Flow Battery Works A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. The ion exchange that occurs between the cathode and anode generates electricity. Most commercial flow batteries. How Flow Battery Works.
From www.xda-developers.com
How does Fast Charging work, and how to use the fastest charging tech How Flow Battery Works A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. During discharge, electrons liberated by reactions on one side travel to the other side. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; The ion exchange that occurs between the cathode and anode. How Flow Battery Works.
From thekflow.blogspot.com
Vanadium redox flow batteries can provide cheap, largescale grid How Flow Battery Works The ion exchange that occurs between the cathode and anode generates electricity. They are rechargeable batteries that separate the energy storage. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. During discharge, electrons liberated by reactions on one side travel to the other side. Flow batteries stand. How Flow Battery Works.
From www.caplinq.com
Batteries Power and Energy CAPLINQ How Flow Battery Works Flow batteries stand out from conventional batteries with their distinct operation and structure. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. The ion exchange that occurs between the cathode and anode generates electricity. They. How Flow Battery Works.
From www.youtube.com
How Does a Flow Battery Work YouTube How Flow Battery Works During discharge, electrons liberated by reactions on one side travel to the other side. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. The flow battery is a form of battery in which electrolyte containing one or. How Flow Battery Works.
From www.slideserve.com
PPT Flow batteries for energy storage PowerPoint Presentation, free How Flow Battery Works Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. During discharge, electrons liberated by reactions on one side travel to the other side. They are rechargeable batteries that separate the energy storage. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; Flow batteries stand out from conventional batteries with. How Flow Battery Works.
From www.researchgate.net
Schematic of the lithium ion battery working principle31. How Flow Battery Works Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; The ion exchange that occurs between the cathode and anode generates electricity. In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active. How Flow Battery Works.
From www.eurekalert.org
How does a flow battery work [IMAGE] EurekAlert! Science News Releases How Flow Battery Works In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. They are rechargeable batteries that separate the energy storage. During discharge, electrons liberated by reactions on one side travel to the other side. The ion exchange that occurs between the cathode and anode generates electricity. Flow batteries, also known as. How Flow Battery Works.
From www.alamy.com
How a lithium ion battery works, 3d render, section. Battery charging How Flow Battery Works The ion exchange that occurs between the cathode and anode generates electricity. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. During discharge, electrons liberated by reactions on one side travel to the other side.. How Flow Battery Works.
From diagramlibrarycoop.z13.web.core.windows.net
Do Batteries Flow From Positive To Negative How Flow Battery Works Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. During discharge, electrons liberated by reactions on one side travel to the other side. The ion exchange that occurs between the cathode and anode generates electricity. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive. How Flow Battery Works.
From www.planete-energies.com
How Do LithiumIon Batteries Work? Planète Énergies How Flow Battery Works In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. Flow batteries stand out from conventional batteries with their distinct operation and structure. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. During discharge, electrons. How Flow Battery Works.
From www.solarreviews.com
What are flow batteries and how do they work? How Flow Battery Works A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. During discharge, electrons liberated by reactions on one side travel to the other side.. How Flow Battery Works.
From cleantechnica.com
ESS Flow Battery To Supply 200MW/2GWh Of Energy Storage To California How Flow Battery Works Flow batteries stand out from conventional batteries with their distinct operation and structure. The ion exchange that occurs between the cathode and anode generates electricity. They are rechargeable batteries that separate the energy storage. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. In a flow battery,. How Flow Battery Works.
From www.tdworld.com
ESS Iron Flow Batteries Getting Installed as Part of SDG&E’s Innovative How Flow Battery Works Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; The ion exchange that occurs between the cathode and. How Flow Battery Works.
From www.brookings.edu
Five emerging battery technologies for electric vehicles How Flow Battery Works The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. The ion exchange that occurs between the cathode and anode generates electricity. Most commercial. How Flow Battery Works.
From www.thermal-engineering.org
Flow Batteries Liquid Electrolytes & Energy Storage How Flow Battery Works They are rechargeable batteries that separate the energy storage. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; The ion exchange that occurs between the cathode and anode generates electricity. During discharge, electrons. How Flow Battery Works.
From www.hisolarener.com
How lithiumion batteries works? HISOLAR New Energy How Flow Battery Works During discharge, electrons liberated by reactions on one side travel to the other side. Flow batteries stand out from conventional batteries with their distinct operation and structure. They are rechargeable batteries that separate the energy storage. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. The flow battery is a form of. How Flow Battery Works.
From www.solarreviews.com
What are flow batteries and how do they work? How Flow Battery Works The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. Flow batteries, also known as redox flow batteries, are designed to store energy in. How Flow Battery Works.
From www.solarreviews.com
What are flow batteries and how do they work? How Flow Battery Works In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. Flow batteries stand out from conventional batteries with their distinct operation and structure. They are rechargeable. How Flow Battery Works.
From batteryworld.varta-automotive.com
How does a car battery work and how is it constructed? How Flow Battery Works Flow batteries stand out from conventional batteries with their distinct operation and structure. The ion exchange that occurs between the cathode and anode generates electricity. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. A flow battery. How Flow Battery Works.
From vsunenergy.com.au
V SUN Energy Renewable Energy from Vanadium Batteries How Flow Battery Works Flow batteries stand out from conventional batteries with their distinct operation and structure. In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; During discharge, electrons liberated by reactions on one side travel to the other side. The. How Flow Battery Works.
From marlonenergystorage.blogspot.com
Marlon's Energy Storage Blog Flow Batteries How Flow Battery Works In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. Flow batteries stand out from conventional batteries with their distinct operation and structure. Most commercial flow batteries use acid sulfur with vanadium salt. How Flow Battery Works.
From www.electricity-magnetism.org
Lithiumion Battery How it works Reaction, Anode & Cathode How Flow Battery Works Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. In a flow battery, negative and positive electrolytes are pumped through separate loops to porous electrodes separated by a membrane. During discharge, electrons liberated by reactions on one side travel to. How Flow Battery Works.
From salgenx.com
SaltWater Flow Battery Topics How Flow Battery Works During discharge, electrons liberated by reactions on one side travel to the other side. A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. Flow batteries stand out from conventional batteries with their distinct operation and structure. In a flow battery, negative and positive electrolytes are. How Flow Battery Works.
From www.solarchoice.net.au
Going with the flow An introduction to redox flow batteries Solar Choice How Flow Battery Works Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; They are rechargeable batteries that separate the energy storage. Flow batteries, also known as redox flow batteries, are designed to store energy in two liquid electrolytes. The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power.. How Flow Battery Works.
From www.electricity-magnetism.org
¿Cómo funcionan las baterías? How Flow Battery Works A flow battery is a fully rechargeable electrical energy storage device where fluids containing the active materials are pumped through a cell, promoting reduction/oxidation. During discharge, electrons liberated by reactions on one side travel to the other side. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; They are rechargeable batteries that separate the energy storage. The. How Flow Battery Works.
From www.aquametals.com
What Are Battery Anode and Cathode Materials? AquaMetals How Flow Battery Works The flow battery is a form of battery in which electrolyte containing one or more dissolved electroactive species flows through a power. They are rechargeable batteries that separate the energy storage. Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; The ion exchange that occurs between the cathode and anode generates electricity. A flow battery is a. How Flow Battery Works.