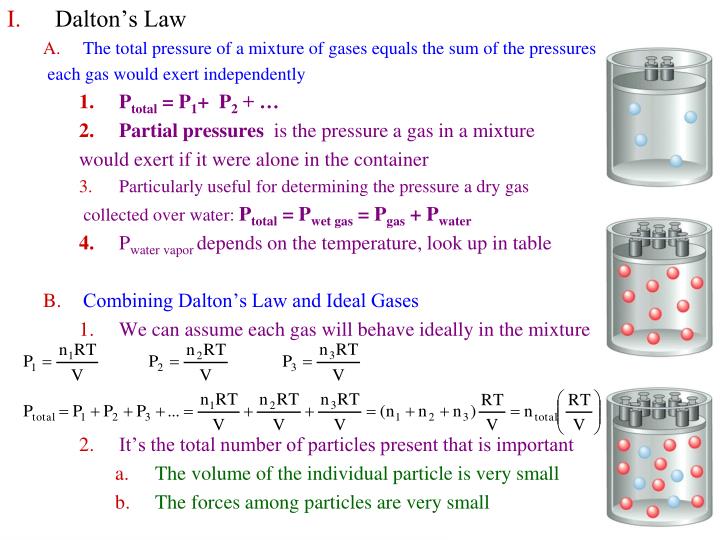
Mixed Gases Pressure . the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. In gas mixtures, each component in the gas phase can be treated separately. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. Each component of the mixture. For a mixture of two. the pressure of a mixture of gases: For a mixture of two. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate.
from www.slideserve.com
the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. Each component of the mixture. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. the pressure of a mixture of gases: — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. For a mixture of two. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. For a mixture of two. In gas mixtures, each component in the gas phase can be treated separately. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate.
PPT Dalton’s Law The total pressure of a mixture of gases equals the
Mixed Gases Pressure In gas mixtures, each component in the gas phase can be treated separately. For a mixture of two. For a mixture of two. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. In gas mixtures, each component in the gas phase can be treated separately. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. the pressure of a mixture of gases: Each component of the mixture.
From www.youtube.com
Gas Density & Average Molar Mass of a Gaseous Mixture, Mole Fraction Mixed Gases Pressure according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. the total pressure of a. Mixed Gases Pressure.
From www.coursehero.com
[Solved] a. A container of mixed gases is 20.9coC and the original Mixed Gases Pressure the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. the pressure of a mixture of gases: — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. For a mixture of two. Each component of the. Mixed Gases Pressure.
From www.slideserve.com
PPT Dalton’s Law The total pressure of a mixture of gases equals the Mixed Gases Pressure For a mixture of two. the pressure of a mixture of gases: — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. For a mixture of two. In gas mixtures, each component in the gas phase can be treated separately. Each component of the mixture. . Mixed Gases Pressure.
From www.edplace.com
Understand Gas Pressure Worksheet EdPlace Mixed Gases Pressure For a mixture of two. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. For a mixture of two. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. In gas mixtures, each. Mixed Gases Pressure.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID943824 Mixed Gases Pressure the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. the pressure of a mixture of gases: For a mixture of two. In gas mixtures, each. Mixed Gases Pressure.
From www.chegg.com
Solved Some N2 gas is mixed with some O2 gas, and the sketch Mixed Gases Pressure — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. the pressure of a mixture of gases: . Mixed Gases Pressure.
From www.slideserve.com
PPT BEHAVIOR OF GASES PowerPoint Presentation, free download ID5765832 Mixed Gases Pressure the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. — by convention, the process of mixing two gases, call them. Mixed Gases Pressure.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Mixed Gases Pressure Each component of the mixture. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. For a mixture of. Mixed Gases Pressure.
From www.yildizgaz.com.tr
Yıldız Gaz Armatürleri Mixed Gas Pressure Regulator with Safety Mixed Gases Pressure For a mixture of two. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. — the pressure exerted by each gas in. Mixed Gases Pressure.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID3233015 Mixed Gases Pressure For a mixture of two. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. the pressure of a mixture of gases: the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. For. Mixed Gases Pressure.
From www.thequizing.com
Determine the average velocity of the mixed gas leaving the tank at a Mixed Gases Pressure For a mixture of two. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. the pressure of a mixture of gases: For a mixture of two. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. —. Mixed Gases Pressure.
From www.coursehero.com
[Solved] thank you so much. Some N, gas is mixed with some O, gas, and Mixed Gases Pressure — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. For a mixture of two. according to dalton’s law of partial pressures, the. Mixed Gases Pressure.
From www.researchgate.net
Effect of CO 2 partial pressure on mixedgas CO 2 /N 2 selectivity in Mixed Gases Pressure — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. For a mixture of two. In gas mixtures, each component in the gas phase. Mixed Gases Pressure.
From www.researchgate.net
Physical properties of gas a phase diagram of pure CO2 Mixed Gases Pressure For a mixture of two. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. For a mixture of two. according to dalton’s law of partial. Mixed Gases Pressure.
From chemistryguru.com.sg
Ideal Gas Law and Applications Mixed Gases Pressure the pressure of a mixture of gases: — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. For a mixture of two. Each component of the mixture. In gas mixtures, each component in the gas phase can be treated separately. the total pressure of a. Mixed Gases Pressure.
From www.chegg.com
Solved 4. Two gases react chemically when they are mixed. Mixed Gases Pressure the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. For a mixture of two. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. In gas mixtures, each component in the gas phase. Mixed Gases Pressure.
From www.slideserve.com
PPT Chapter 11 Gases PowerPoint Presentation, free download ID64297 Mixed Gases Pressure the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the. Mixed Gases Pressure.
From chemistryguru.com.sg
Ideal Gas Law and Applications Mixed Gases Pressure the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which. Mixed Gases Pressure.
From mmerevise.co.uk
The Ideal Gas Equation MME Mixed Gases Pressure Each component of the mixture. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. In gas mixtures, each component in the gas phase can be treated separately. the pressure of a mixture of gases: the total pressure of a mixture of gases is the. Mixed Gases Pressure.
From owlcation.com
The Theories and Behavior of Gas Owlcation Mixed Gases Pressure — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. In gas mixtures, each component in the gas phase can be treated separately. For a mixture of two. the total pressure of a mixture of gases is the sum of the partial pressures. Mixed Gases Pressure.
From sciencenotes.org
Ideal Gas Law Formula and Examples Mixed Gases Pressure — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. For a mixture of two. In gas mixtures, each component in the gas phase can be treated separately. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the. Mixed Gases Pressure.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec Mixed Gases Pressure Each component of the mixture. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. — the pressure exerted by each. Mixed Gases Pressure.
From www.chegg.com
Solved Some N2 gas is mixed with some 02 gas, and the sketch Mixed Gases Pressure — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. the pressure of a mixture of gases: — the pressure exerted by. Mixed Gases Pressure.
From drcalef.com
The Combined Gas Law Pressure, Volume, and Temperature Mixed Gases Pressure For a mixture of two. For a mixture of two. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. the pressure of a mixture of gases: the total pressure of a mixture of gases is the sum of the. Mixed Gases Pressure.
From www.youtube.com
Ideal Gas Law Total Pressure of Two Flasks YouTube Mixed Gases Pressure For a mixture of two. In gas mixtures, each component in the gas phase can be treated separately. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two. Mixed Gases Pressure.
From gulppp.com
PSI Pressure Calculation Chart For Mixed Gas 30 CO2 and 70 Nitrogen Mixed Gases Pressure For a mixture of two. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. In gas mixtures, each component in the gas phase can be treated separately. the pressure of a mixture of gases: — the pressure exerted by each gas in a gas mixture (its partial. Mixed Gases Pressure.
From www.chegg.com
Solved Two gases are contained in the apparatus below, one Mixed Gases Pressure — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. For a mixture of two. Each component of the mixture. the pressure of a mixture of gases: according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the. Mixed Gases Pressure.
From www.slideserve.com
PPT Gas MixturesPartial Pressure PowerPoint Presentation, free Mixed Gases Pressure In gas mixtures, each component in the gas phase can be treated separately. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted. Mixed Gases Pressure.
From www.tec-science.com
Thermal conductivity of gases tecscience Mixed Gases Pressure according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. For a mixture of two. — the pressure exerted by each. Mixed Gases Pressure.
From www.chegg.com
Solved Oxygen and methane are mixed at 200 kPa abs pressure Mixed Gases Pressure the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. In gas mixtures, each component in the gas phase can be treated separately. For a mixture of. Mixed Gases Pressure.
From www.toppr.com
The pressure and temperature of two different gases is P and T having Mixed Gases Pressure — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. For a mixture of two. For a mixture of two. the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. according to dalton’s. Mixed Gases Pressure.
From www.youtube.com
Ideal gas mixture mixing two tanks YouTube Mixed Gases Pressure the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. In gas mixtures, each component in the gas phase can be treated separately. For a mixture of two. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two. Mixed Gases Pressure.
From wisc.pb.unizin.org
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Mixed Gases Pressure — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. according to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. In gas mixtures, each component in the gas phase. Mixed Gases Pressure.
From www.youtube.com
two ideal gases mix YouTube Mixed Gases Pressure the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. — the pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all. the pressure of a mixture of gases: the total pressure of a mixture of gases. Mixed Gases Pressure.
From saylordotorg.github.io
Gases and Pressure Mixed Gases Pressure the pressure of a mixture of gases: Each component of the mixture. — by convention, the process of mixing two gases, call them \(a\) and \(b\), is the process in which the two gases initially occupy separate. For a mixture of two. the total pressure of a mixture of gases is the sum of the partial pressures. Mixed Gases Pressure.