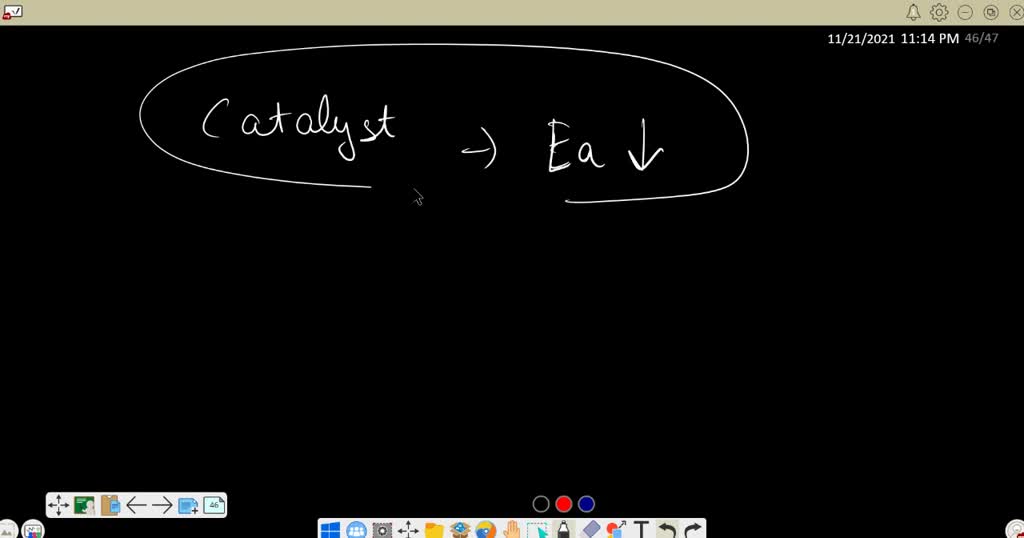Catalyst Decreases The Activation Energy Of . estimate the activation energy for each process, and identify which one involves a catalyst. a catalyst speeds up the rate of a reaction by lowering the activation energy; In contrast, a negative catalyst makes a reaction. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a positive catalyst lowers the activation energy of a reaction and speeds up its rate. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for the. for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. In addition, the catalyst is regenerated in the process.
from www.numerade.com
to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for the. estimate the activation energy for each process, and identify which one involves a catalyst. for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a catalyst speeds up the rate of a reaction by lowering the activation energy; catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. In addition, the catalyst is regenerated in the process. In contrast, a negative catalyst makes a reaction. a positive catalyst lowers the activation energy of a reaction and speeds up its rate.
A catalyst (a) increases the average energy of reacting
Catalyst Decreases The Activation Energy Of In addition, the catalyst is regenerated in the process. a catalyst speeds up the rate of a reaction by lowering the activation energy; for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. In contrast, a negative catalyst makes a reaction. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for the. estimate the activation energy for each process, and identify which one involves a catalyst. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at In addition, the catalyst is regenerated in the process.
From slideplayer.com
Chapter 2 Chemistry of Life 2.1 Atoms, Ions, and Molecules ppt download Catalyst Decreases The Activation Energy Of catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. In contrast, a negative catalyst makes a reaction. estimate the activation energy for each process, and identify which one involves. Catalyst Decreases The Activation Energy Of.
From www.toppr.com
The activation energy of a certain uncatalysed reaction at 300K is Catalyst Decreases The Activation Energy Of a catalyst speeds up the rate of a reaction by lowering the activation energy; for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. estimate the activation energy for each process, and identify which one involves a catalyst. to illustrate how a catalyst can decrease the. Catalyst Decreases The Activation Energy Of.
From www.toppr.com
14 A catalyst (A) Increases the energy change in the reaction (B Catalyst Decreases The Activation Energy Of estimate the activation energy for each process, and identify which one involves a catalyst. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. In contrast, a negative catalyst makes a reaction. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction,. Catalyst Decreases The Activation Energy Of.
From sciencenotes.org
What Is Activation Energy? Definition and Examples Catalyst Decreases The Activation Energy Of In addition, the catalyst is regenerated in the process. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. catalysts are defined as substances that participate in a chemical reaction but are not. Catalyst Decreases The Activation Energy Of.
From www.numerade.com
SOLVED20. [1 mark] What decreases the activation energy ofa reaction Catalyst Decreases The Activation Energy Of In addition, the catalyst is regenerated in the process. In contrast, a negative catalyst makes a reaction. estimate the activation energy for each process, and identify which one involves a catalyst. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. a catalyst speeds up the rate of a reaction. Catalyst Decreases The Activation Energy Of.
From cheminfo.uz
Kataliz va katalizator haqida ChemInfo.uz Catalyst Decreases The Activation Energy Of Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a catalyst speeds up the rate of a reaction by lowering the activation energy; to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for the. a. Catalyst Decreases The Activation Energy Of.
From kunduz.com
[ANSWERED] 4 A catalyst lowers the activation energy of a certain Kunduz Catalyst Decreases The Activation Energy Of a positive catalyst lowers the activation energy of a reaction and speeds up its rate. for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. estimate the activation energy for each process, and identify which one involves a catalyst. In addition, the catalyst is regenerated in the. Catalyst Decreases The Activation Energy Of.
From www.numerade.com
SOLVED Question 4 (1 point) Which of the following statements about Catalyst Decreases The Activation Energy Of a catalyst speeds up the rate of a reaction by lowering the activation energy; a positive catalyst lowers the activation energy of a reaction and speeds up its rate. estimate the activation energy for each process, and identify which one involves a catalyst. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur. Catalyst Decreases The Activation Energy Of.
From byjus.com
What does a negative activation energy mean? Catalyst Decreases The Activation Energy Of for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. estimate the activation energy for each process, and identify which one involves a catalyst. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at In contrast, a negative catalyst makes a reaction. . Catalyst Decreases The Activation Energy Of.
From phys.org
New catalyst decreases the energy required to split hydrogen gas from water Catalyst Decreases The Activation Energy Of for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. In contrast, a negative catalyst makes a reaction. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at to illustrate how a catalyst can decrease the activation energy for a reaction by providing. Catalyst Decreases The Activation Energy Of.
From www.chegg.com
Solved A catalyst decreases the activation energy of a Catalyst Decreases The Activation Energy Of for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for the. a positive catalyst lowers the activation energy of a reaction. Catalyst Decreases The Activation Energy Of.
From gioaralqv.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction In Such A Manner Catalyst Decreases The Activation Energy Of a catalyst speeds up the rate of a reaction by lowering the activation energy; for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at. Catalyst Decreases The Activation Energy Of.
From www.numerade.com
A catalyst (a) increases the average energy of reacting Catalyst Decreases The Activation Energy Of a positive catalyst lowers the activation energy of a reaction and speeds up its rate. estimate the activation energy for each process, and identify which one involves a catalyst. a catalyst speeds up the rate of a reaction by lowering the activation energy; for cellular reactions to occur fast enough over short time scales, their activation. Catalyst Decreases The Activation Energy Of.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyst Decreases The Activation Energy Of a positive catalyst lowers the activation energy of a reaction and speeds up its rate. In contrast, a negative catalyst makes a reaction. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for the. for cellular reactions to occur fast enough. Catalyst Decreases The Activation Energy Of.
From exyepzauq.blob.core.windows.net
Enzymes Help To Lower The Activation Energies Of Reaction By at Laura Catalyst Decreases The Activation Energy Of estimate the activation energy for each process, and identify which one involves a catalyst. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. a positive catalyst lowers the activation energy of. Catalyst Decreases The Activation Energy Of.
From www.edukate.me
Explain The Effect of Catalyst on the Rate of Reaction A catalyst Catalyst Decreases The Activation Energy Of In addition, the catalyst is regenerated in the process. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for the. In contrast, a negative catalyst makes a reaction. a catalyst speeds up the rate of a reaction by lowering the activation energy;. Catalyst Decreases The Activation Energy Of.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Decreases The Activation Energy Of a positive catalyst lowers the activation energy of a reaction and speeds up its rate. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. In addition, the catalyst is regenerated in the process. In contrast, a negative catalyst makes a reaction. Several reactions that are thermodynamically favorable in the absence. Catalyst Decreases The Activation Energy Of.
From www.numerade.com
SOLVED20. [1 mark] What decreases the activation energy ofa reaction Catalyst Decreases The Activation Energy Of In contrast, a negative catalyst makes a reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. In addition, the catalyst is regenerated in the process. for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. to illustrate how. Catalyst Decreases The Activation Energy Of.
From byjus.com
ntIf activation energy of reaction decreases by 5.727kJ when catalyst Catalyst Decreases The Activation Energy Of catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. estimate the activation energy for each process, and identify which one involves a catalyst. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for. Catalyst Decreases The Activation Energy Of.
From www.chegg.com
Solved A catalyst decreases the activation energy of a Catalyst Decreases The Activation Energy Of Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a positive catalyst lowers the activation energy of a reaction and speeds up its rate. In addition, the catalyst is regenerated in the process. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. for. Catalyst Decreases The Activation Energy Of.
From brainly.com
Which statement best describes how a catalyst can speed up a chemical Catalyst Decreases The Activation Energy Of for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a catalyst speeds up the rate of a reaction by lowering the activation energy; a positive catalyst lowers the activation energy of. Catalyst Decreases The Activation Energy Of.
From 2012books.lardbucket.org
Catalysis Catalyst Decreases The Activation Energy Of a catalyst speeds up the rate of a reaction by lowering the activation energy; In addition, the catalyst is regenerated in the process. for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. a positive catalyst lowers the activation energy of a reaction and speeds up its. Catalyst Decreases The Activation Energy Of.
From brainly.com
A catalyst decreases the activation energy of a particular exothermic Catalyst Decreases The Activation Energy Of a catalyst speeds up the rate of a reaction by lowering the activation energy; a positive catalyst lowers the activation energy of a reaction and speeds up its rate. for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. estimate the activation energy for each process,. Catalyst Decreases The Activation Energy Of.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Catalyst Decreases The Activation Energy Of for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at estimate the activation energy for. Catalyst Decreases The Activation Energy Of.
From www.chegg.com
Solved A catalyst decreases the activation energy of a Catalyst Decreases The Activation Energy Of a catalyst speeds up the rate of a reaction by lowering the activation energy; for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at estimate the activation energy for each process, and. Catalyst Decreases The Activation Energy Of.
From byjus.com
How does a catalyst increase the rate of a reaction? Catalyst Decreases The Activation Energy Of estimate the activation energy for each process, and identify which one involves a catalyst. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. In contrast, a negative catalyst makes a reaction. . Catalyst Decreases The Activation Energy Of.
From www.doubtnut.com
X = energy of activation with catalyst, Y = energy of activation wit Catalyst Decreases The Activation Energy Of Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at In contrast, a negative catalyst makes a reaction. a catalyst speeds up the rate of a reaction by lowering the activation energy; a positive catalyst lowers the activation energy of a reaction and speeds up its rate. In addition, the catalyst is regenerated. Catalyst Decreases The Activation Energy Of.
From www.numerade.com
SOLVED 'A catalyst decreases the activation energy of a given reaction Catalyst Decreases The Activation Energy Of for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. a positive catalyst lowers the. Catalyst Decreases The Activation Energy Of.
From www.chim.lu
Activation energy and catalysis Catalyst Decreases The Activation Energy Of a positive catalyst lowers the activation energy of a reaction and speeds up its rate. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. In contrast, a negative catalyst makes a reaction. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway. Catalyst Decreases The Activation Energy Of.
From giorbnkft.blob.core.windows.net
Catalyst Chemistry Term at Nancy Sherry blog Catalyst Decreases The Activation Energy Of a catalyst speeds up the rate of a reaction by lowering the activation energy; a positive catalyst lowers the activation energy of a reaction and speeds up its rate. In addition, the catalyst is regenerated in the process. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. for. Catalyst Decreases The Activation Energy Of.
From www.chegg.com
Solved 15.If a catalyst decreases the activation energy of a Catalyst Decreases The Activation Energy Of a positive catalyst lowers the activation energy of a reaction and speeds up its rate. estimate the activation energy for each process, and identify which one involves a catalyst. a catalyst speeds up the rate of a reaction by lowering the activation energy; to illustrate how a catalyst can decrease the activation energy for a reaction. Catalyst Decreases The Activation Energy Of.
From www.numerade.com
SOLVED By which of the following mechanisms does a catalyst operate Catalyst Decreases The Activation Energy Of In addition, the catalyst is regenerated in the process. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for the. Several reactions that are thermodynamically. Catalyst Decreases The Activation Energy Of.
From www.chegg.com
Solved A catalyst decreases the activation energy of a Catalyst Decreases The Activation Energy Of Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. In contrast, a negative catalyst makes a reaction. a catalyst speeds up the rate of a reaction by lowering the activation energy; . Catalyst Decreases The Activation Energy Of.
From www.numerade.com
SOLVED The presence of a catalyst decreases the activation energy of a Catalyst Decreases The Activation Energy Of In addition, the catalyst is regenerated in the process. a catalyst speeds up the rate of a reaction by lowering the activation energy; In contrast, a negative catalyst makes a reaction. to illustrate how a catalyst can decrease the activation energy for a reaction by providing another pathway for the reaction, let's look at the mechanism for the.. Catalyst Decreases The Activation Energy Of.
From www.chegg.com
Solved 20. [1 mark] What decreases the activation energy of Catalyst Decreases The Activation Energy Of catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. estimate the activation energy for each process, and identify which one involves a catalyst. for cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. a positive catalyst lowers the. Catalyst Decreases The Activation Energy Of.