Quality Control Laboratory Layout . Under this approach the design and number of control areas are. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Use control areas as described in the nycfc instead of laboratory units. The ispe good practice guide: A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Quality and compliance in quality control laboratories primary objectives of regulatory.
from www.gmpsop.com
Quality and compliance in quality control laboratories primary objectives of regulatory. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Under this approach the design and number of control areas are. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. The ispe good practice guide: Use control areas as described in the nycfc instead of laboratory units.
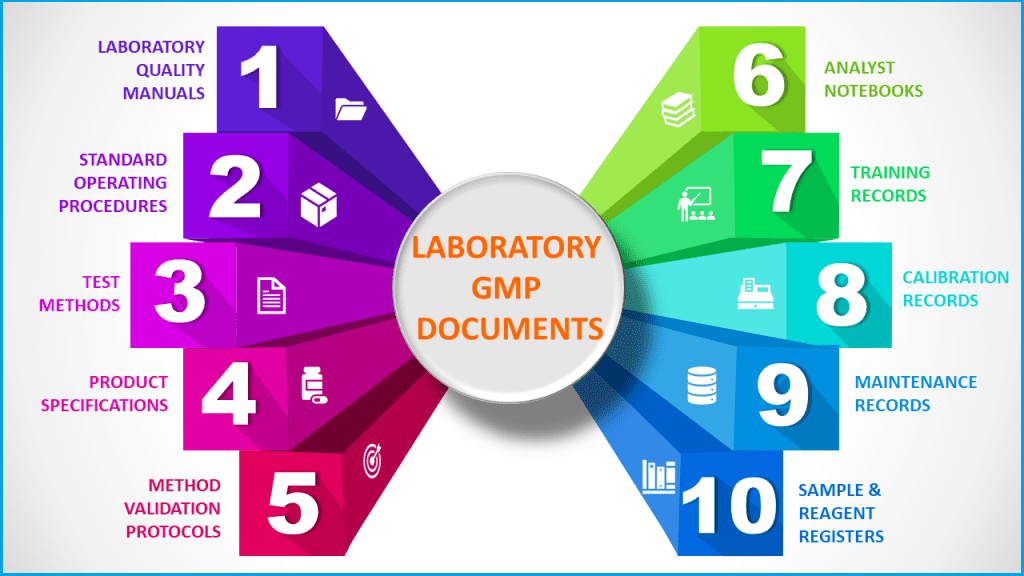
Typical GMP documentation in a quality control laboratory
Quality Control Laboratory Layout Under this approach the design and number of control areas are. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Under this approach the design and number of control areas are. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. The ispe good practice guide: Quality and compliance in quality control laboratories primary objectives of regulatory. Use control areas as described in the nycfc instead of laboratory units.
From www.hemcocorp.com
Modular Labs Quality Control Laboratory Layout Quality and compliance in quality control laboratories primary objectives of regulatory. Use control areas as described in the nycfc instead of laboratory units. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Inspections of. Quality Control Laboratory Layout.
From www.brewersassociation.org
Design and Construction of Brewery Quality Labs Brewers Association Quality Control Laboratory Layout Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. The ispe good practice guide: A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Under this. Quality Control Laboratory Layout.
From www.gmpsop.com
Typical GMP documentation in a quality control laboratory Quality Control Laboratory Layout Under this approach the design and number of control areas are. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. The ispe good practice guide: Inspections of sites involved in testing of medicinal products should be. Quality Control Laboratory Layout.
From architizer.com
Teva Quality control laboratory and microbiology by Segal Vissotto Quality Control Laboratory Layout Quality and compliance in quality control laboratories primary objectives of regulatory. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. The ispe good practice guide: Under this approach the design and number of control areas. Quality Control Laboratory Layout.
From satoriseal.com
Quality Control Laboratory Testing Physical Property of Rubber Quality Control Laboratory Layout The ispe good practice guide: This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Quality. Quality Control Laboratory Layout.
From www.crbgroup.com
5 considerations for laboratory site selection CRB Quality Control Laboratory Layout Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Use control areas as described in the nycfc instead of laboratory units. Inspections of sites involved in testing of medicinal products should be more and more. Quality Control Laboratory Layout.
From www.dreamstime.com
Quality control lab stock photo. Image of scientific, laboratory 2305384 Quality Control Laboratory Layout Quality and compliance in quality control laboratories primary objectives of regulatory. Use control areas as described in the nycfc instead of laboratory units. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Inspections of sites. Quality Control Laboratory Layout.
From coolcolorsinphotography.blogspot.com
laboratory layout design software coolcolorsinphotography Quality Control Laboratory Layout The ispe good practice guide: Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Under this approach the design and number of control areas are. Quality and compliance in quality control laboratories primary objectives of regulatory. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and. Quality Control Laboratory Layout.
From hootoh.my
Effective Medical Laboratory Layout for Optimal Performance Quality Control Laboratory Layout Quality and compliance in quality control laboratories primary objectives of regulatory. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Use control areas as described in the nycfc instead of laboratory units. Under this approach the design and number of control areas are. The ispe good practice guide: Inspections of sites. Quality Control Laboratory Layout.
From www.pinterest.com
The layout of the laboratory must be sensibly planned and logically Quality Control Laboratory Layout Under this approach the design and number of control areas are. Use control areas as described in the nycfc instead of laboratory units. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. The ispe good practice guide: A comprehensive guide to the design and renovation of laboratories, covering health, safety,. Quality Control Laboratory Layout.
From www.researchgate.net
Layout of clinical laboratory. Download Scientific Diagram Quality Control Laboratory Layout The ispe good practice guide: This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Quality and compliance in quality control laboratories primary objectives of regulatory. Under this approach the design and number of control areas are. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations.. Quality Control Laboratory Layout.
From dxofmztzw.blob.core.windows.net
Guidelines For Food Laboratory at Bradley Fox blog Quality Control Laboratory Layout Quality and compliance in quality control laboratories primary objectives of regulatory. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. The ispe good practice guide: This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. A comprehensive guide to the design and renovation of. Quality Control Laboratory Layout.
From architizer.com
Teva Quality control laboratory and microbiology by Segal Vissotto Quality Control Laboratory Layout Under this approach the design and number of control areas are. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Use control areas as described in the nycfc instead of laboratory units. The ispe good practice guide: This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin. Quality Control Laboratory Layout.
From www.chef-wan.com.my
The Best Part of Laboratory Equipment Malaysia Chef Wan Quality Control Laboratory Layout Under this approach the design and number of control areas are. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. A comprehensive guide to the design and renovation of laboratories, covering health,. Quality Control Laboratory Layout.
From mcgrathconstruction.com
McGrath & Associates begins work on new quality control laboratory Quality Control Laboratory Layout Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Under this approach the design and number of control areas are. Use control areas as described in the nycfc instead of laboratory units. The. Quality Control Laboratory Layout.
From viewfloor.co
Clinical Laboratory Floor Plan With Workflow Viewfloor.co Quality Control Laboratory Layout Under this approach the design and number of control areas are. The ispe good practice guide: Quality and compliance in quality control laboratories primary objectives of regulatory. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Inspections of sites involved in testing of medicinal products should be more and more specific,. Quality Control Laboratory Layout.
From shrijeelifestyle.com
Quality Control State Of The Art Lab, Fabric Testing Laboratory, Mumbai Quality Control Laboratory Layout This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Quality and compliance in quality control laboratories primary objectives of regulatory. The ispe good practice guide: Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Use control areas as described in the nycfc instead. Quality Control Laboratory Layout.
From www.complianceonline.com
cGMP and GLP Regulations for Quality Control Labs An overview Quality Control Laboratory Layout Use control areas as described in the nycfc instead of laboratory units. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. The ispe good practice guide: Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. A comprehensive guide to the design. Quality Control Laboratory Layout.
From www.flad.com
Takeda Pharmaceutical Company Limited Quality Control Laboratory Quality Control Laboratory Layout Use control areas as described in the nycfc instead of laboratory units. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. Under this approach the design and number of control areas are. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. This document. Quality Control Laboratory Layout.
From www.slideserve.com
PPT Laboratory Quality Control PowerPoint Presentation, free download Quality Control Laboratory Layout This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Use control areas as described in the nycfc instead of laboratory units. Under this approach the design and number of control areas are. The ispe good practice guide: Annex 1 provides advice on the quality management system and practices for testing apis, excipients. Quality Control Laboratory Layout.
From manoxblog.com
Pharmaceutical Quality Control Laboratory layout M A N O X B L O G Quality Control Laboratory Layout A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. Under this approach the design and number of control areas are. Annex 1 provides advice on the quality management system and practices for testing apis,. Quality Control Laboratory Layout.
From cutegirlbabywallpaperhd.blogspot.com
laboratory layout design software cutegirlbabywallpaperhd Quality Control Laboratory Layout Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Under this approach the design and number. Quality Control Laboratory Layout.
From viewfloor.co
Floor Plan Laboratory Layout Drawing Viewfloor.co Quality Control Laboratory Layout Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Use control areas as described in the nycfc. Quality Control Laboratory Layout.
From www.researchgate.net
Floor plan of the laboratory room where the measurements took place Quality Control Laboratory Layout Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. A comprehensive guide to the design. Quality Control Laboratory Layout.
From www.researchgate.net
Floor plans for three different 40 ft/12 m container conversions Quality Control Laboratory Layout Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Under this approach the design and number of control areas are. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. This document provides good practices for laboratories involved in sterility testing, microbial. Quality Control Laboratory Layout.
From www.slideshare.net
Quality control in the medical laboratory Quality Control Laboratory Layout The ispe good practice guide: Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Under this approach the design and number of control areas are. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Quality and compliance in quality control laboratories primary objectives. Quality Control Laboratory Layout.
From www.gfenergy.gr
qualitycontrollab GF Energy Quality Control Laboratory Layout Under this approach the design and number of control areas are. Quality and compliance in quality control laboratories primary objectives of regulatory. A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Use control areas as described in the nycfc instead of laboratory units. Inspections of sites involved in testing of medicinal products should. Quality Control Laboratory Layout.
From dribbble.com
Testing Laboratory Layout Template by Icograms on Dribbble Quality Control Laboratory Layout The ispe good practice guide: Quality and compliance in quality control laboratories primary objectives of regulatory. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Use control areas as described in the nycfc instead of laboratory units. Annex 1 provides advice on the quality management system and practices for testing apis, excipients. Quality Control Laboratory Layout.
From www.labs31.com
News Seminar 4D laboratory Quality Control Laboratory Layout Quality and compliance in quality control laboratories primary objectives of regulatory. Under this approach the design and number of control areas are. Use control areas as described in the nycfc instead of laboratory units. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. A comprehensive guide to the design and renovation. Quality Control Laboratory Layout.
From www.researchgate.net
Layout of a biosafety level 3 container laboratory. Download Quality Control Laboratory Layout This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. The ispe good practice guide: Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. Use control areas as described in the nycfc instead of laboratory units. Quality and compliance in quality control laboratories. Quality Control Laboratory Layout.
From present5.com
Module 12 Quality Control The Lab Quality Quality Control Laboratory Layout The ispe good practice guide: A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. Quality and compliance in quality control laboratories primary objectives of regulatory. Annex 1 provides advice on the quality management system and practices. Quality Control Laboratory Layout.
From www.testextextile.com
Step By Step To Build A Complete Textile Quality Control Laboratory Quality Control Laboratory Layout Use control areas as described in the nycfc instead of laboratory units. Annex 1 provides advice on the quality management system and practices for testing apis, excipients and pharmaceutical products. Quality and compliance in quality control laboratories primary objectives of regulatory. Under this approach the design and number of control areas are. A comprehensive guide to the design and renovation. Quality Control Laboratory Layout.
From www.velvetjobs.com
Quality Control Laboratory Job Description Velvet Jobs Quality Control Laboratory Layout Under this approach the design and number of control areas are. The ispe good practice guide: A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Quality and compliance in quality control laboratories primary objectives of regulatory. Use control areas as described in the nycfc instead of laboratory units. Inspections of sites involved in. Quality Control Laboratory Layout.
From manoxblog.com
Pharmaceutical Quality Control Laboratory Layout M A N O X B L O G Quality Control Laboratory Layout A comprehensive guide to the design and renovation of laboratories, covering health, safety, and environmental considerations. Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. The ispe good practice guide: Annex 1 provides. Quality Control Laboratory Layout.
From www.re-thinkingthefuture.com
An overview of Laboratory design RTF Rethinking The Future Quality Control Laboratory Layout Inspections of sites involved in testing of medicinal products should be more and more specific, thorough and conducted under. Quality and compliance in quality control laboratories primary objectives of regulatory. This document provides good practices for laboratories involved in sterility testing, microbial detection, identification and endotoxin testing. A comprehensive guide to the design and renovation of laboratories, covering health, safety,. Quality Control Laboratory Layout.