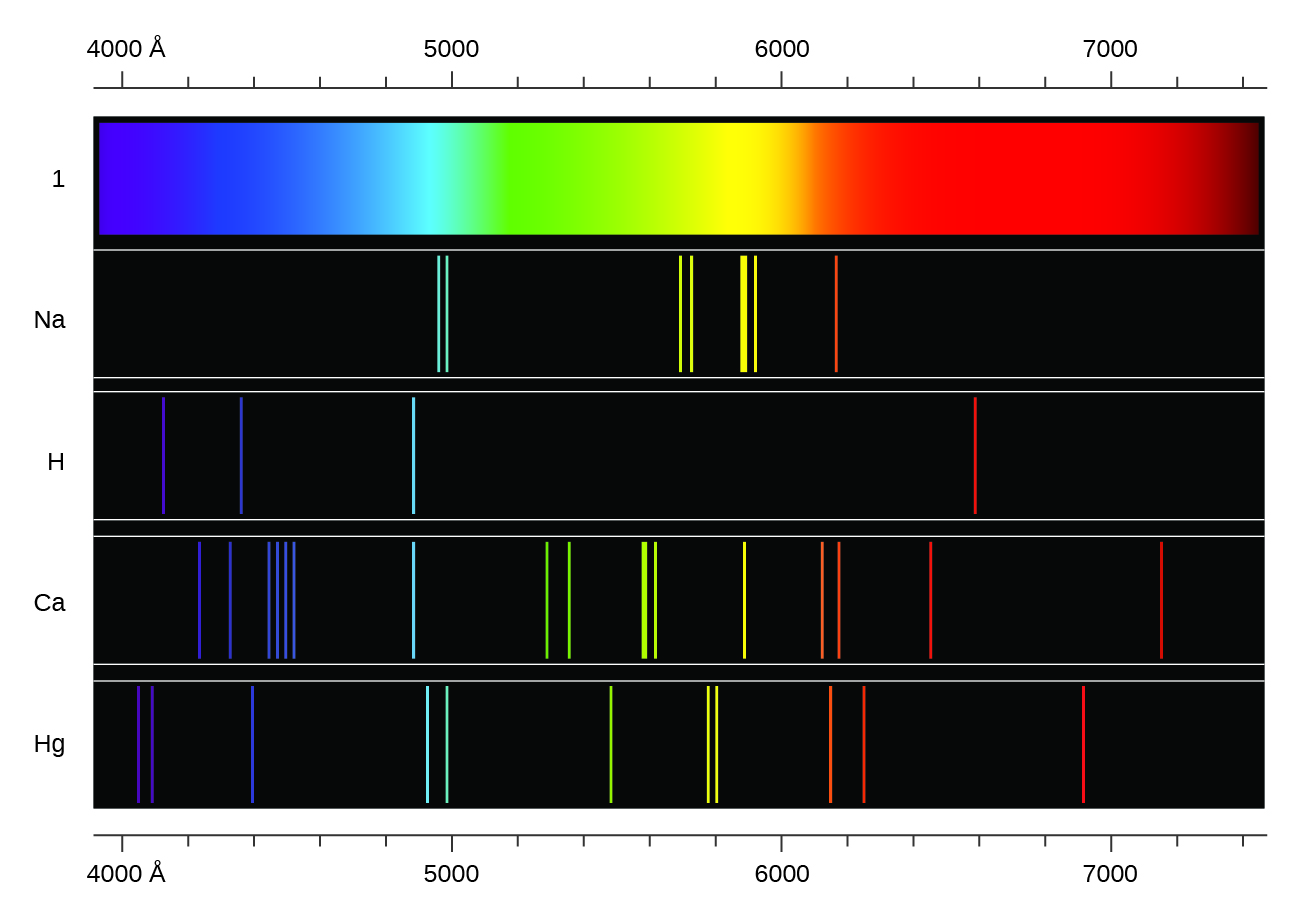
Emission Spectra Example . For example, by studying emission spectra of the stars, we can determine their chemical composition. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. Identifying an unknown gas using emission spectra. In order to identify the gas, he. Also, emission spectra are used. A scientist has a sample of an unknown gas. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum.
from wisc.pb.unizin.org
A scientist has a sample of an unknown gas. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. In order to identify the gas, he. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Also, emission spectra are used. Identifying an unknown gas using emission spectra. For example, by studying emission spectra of the stars, we can determine their chemical composition.
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/
Emission Spectra Example In order to identify the gas, he. Identifying an unknown gas using emission spectra. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. A scientist has a sample of an unknown gas. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used. In order to identify the gas, he. The result is an emission spectrum that shows the intensity of emission as a function of wavelength.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectra Example Also, emission spectra are used. A scientist has a sample of an unknown gas. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. For example, by studying emission spectra of the stars, we can determine their chemical composition. Emission and absorption spectra form the basis of spectroscopy, which. Emission Spectra Example.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Example Identifying an unknown gas using emission spectra. For example, by studying emission spectra of the stars, we can determine their chemical composition. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Also, emission spectra are used. A scientist has a sample of an unknown gas. The result is an emission. Emission Spectra Example.
From www.researchgate.net
Spectra of four types of galaxies (Terlevich et al Emission Spectra Example The result is an emission spectrum that shows the intensity of emission as a function of wavelength. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Identifying an unknown gas using emission spectra. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the.. Emission Spectra Example.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Example This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Also, emission spectra are used. For example, by studying emission spectra of the stars, we can determine their chemical composition. The electromagnetic spectrum was. Emission Spectra Example.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Example Also, emission spectra are used. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In order to identify the gas, he. Identifying an unknown gas using emission spectra. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. A scientist has a sample. Emission Spectra Example.
From winstonmcyponce.blogspot.com
Atomic Emission Spectrum of Hydrogen WinstonmcyPonce Emission Spectra Example Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. For example, by studying emission. Emission Spectra Example.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra Example Identifying an unknown gas using emission spectra. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Also, emission spectra are used. This spectrum of radiation emitted by electrons in the excited atoms or molecules is. Emission Spectra Example.
From www.slideserve.com
PPT Chapter 42 PowerPoint Presentation, free download ID5580472 Emission Spectra Example A scientist has a sample of an unknown gas. In order to identify the gas, he. For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. The result is an emission spectrum that. Emission Spectra Example.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Example This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. Also, emission spectra. Emission Spectra Example.
From mavink.com
Visible Line Spectrum Of Hydrogen Emission Spectra Example Identifying an unknown gas using emission spectra. For example, by studying emission spectra of the stars, we can determine their chemical composition. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. In order to identify. Emission Spectra Example.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Example The result is an emission spectrum that shows the intensity of emission as a function of wavelength. For example, by studying emission spectra of the stars, we can determine their chemical composition. Identifying an unknown gas using emission spectra. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Also, emission. Emission Spectra Example.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Emission Spectra Example Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. A scientist has a sample of an unknown gas. For example, by studying emission spectra of the stars, we can determine their chemical composition. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves,. Emission Spectra Example.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Emission Spectra Example For example, by studying emission spectra of the stars, we can determine their chemical composition. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Also, emission spectra are used. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Identifying an unknown gas. Emission Spectra Example.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectra Example Also, emission spectra are used. Identifying an unknown gas using emission spectra. In order to identify the gas, he. For example, by studying emission spectra of the stars, we can determine their chemical composition. A scientist has a sample of an unknown gas. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the.. Emission Spectra Example.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectra Example Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. For example, by studying emission spectra of the stars, we can determine their chemical composition. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The electromagnetic spectrum was divided into regions, many of. Emission Spectra Example.
From stock.adobe.com
Continuous spectrum, an emission spectrum that consists of continuum of Emission Spectra Example This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. For example, by studying emission spectra of the stars, we can determine their chemical composition. Identifying an unknown gas using emission spectra. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. The result. Emission Spectra Example.
From www.pinterest.com
Absorption spectrum of the elements spectroscopy Physics, Earth and Emission Spectra Example Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used. In order to identify the gas,. Emission Spectra Example.
From www.researchgate.net
A typical example showing the Gaussian fitting of the emission spectra Emission Spectra Example In order to identify the gas, he. For example, by studying emission spectra of the stars, we can determine their chemical composition. Identifying an unknown gas using emission spectra. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. This spectrum of radiation emitted by electrons in the excited. Emission Spectra Example.
From www.esa.int
ESA Absorption and emission spectra of various elements Emission Spectra Example The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Identifying an unknown gas using emission spectra. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. For example, by studying emission spectra of the stars, we can determine their chemical composition.. Emission Spectra Example.
From www.slideserve.com
PPT Lecture 15 The Hydrogen Atom PowerPoint Presentation ID2710708 Emission Spectra Example The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. A scientist has a sample of an unknown gas. Identifying an unknown gas using emission spectra. The electromagnetic spectrum was divided into regions, many of which. Emission Spectra Example.
From www.youtube.com
2.2 Hydrogen emission spectrum (SL) YouTube Emission Spectra Example This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. For example, by studying emission spectra of the stars, we can determine their chemical composition. Also, emission spectra are used. In order to identify the gas, he. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide. Emission Spectra Example.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Example Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Identifying an unknown gas using emission spectra. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. For example, by studying emission spectra of the stars, we can determine their chemical composition. The result. Emission Spectra Example.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Emission Spectra Example For example, by studying emission spectra of the stars, we can determine their chemical composition. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In order to identify the gas, he. A scientist has a sample of an unknown gas. The electromagnetic spectrum was divided into regions, many of which. Emission Spectra Example.
From www.slideshare.net
Chapter 3 Arrangement of Electrons in the Atom Emission Spectra Example A scientist has a sample of an unknown gas. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. In order to identify the gas, he. For example, by studying emission spectra of the. Emission Spectra Example.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Emission Spectra Example This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. Also, emission spectra are used. The result is an emission spectrum that shows the intensity of emission as a function. Emission Spectra Example.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra Example Identifying an unknown gas using emission spectra. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Also, emission spectra are used. A scientist has a sample of an unknown gas. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. In. Emission Spectra Example.
From www.pinterest.jp
Emission Spectra of the Elements!! Chemistry classroom, Teaching Emission Spectra Example A scientist has a sample of an unknown gas. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves,. Identifying an unknown gas using emission spectra. Also, emission spectra are used. This. Emission Spectra Example.
From www.slideserve.com
PPT Chapter 42 PowerPoint Presentation, free download ID5580472 Emission Spectra Example This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Identifying an unknown gas using emission spectra. A scientist has a sample of an unknown gas. For example, by studying emission spectra of the stars, we can determine their chemical composition. The result is an emission spectrum that shows the intensity. Emission Spectra Example.
From www.researchgate.net
Normalised (A) absorption and (B) emission spectra of the fluorescent Emission Spectra Example This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Identifying an unknown gas using emission spectra. A scientist has a sample of an unknown gas. For example, by studying emission spectra of the stars, we can determine their chemical composition. Emission and absorption spectra form the basis of spectroscopy, which. Emission Spectra Example.
From sixdayscience.com
Chapter 7 SixDay Science Emission Spectra Example A scientist has a sample of an unknown gas. For example, by studying emission spectra of the stars, we can determine their chemical composition. In order to identify the gas, he. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. This spectrum of radiation emitted by electrons in the excited atoms or. Emission Spectra Example.
From www.visionlearning.com
History of Earth's Atmosphere I Earth Science Visionlearning Emission Spectra Example Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. A scientist has a sample of an unknown gas. Identifying an unknown gas using emission spectra. For example, by studying emission spectra of the. Emission Spectra Example.
From www.anisotropela.dk
Redshift Emission Spectra Example The result is an emission spectrum that shows the intensity of emission as a function of wavelength. In order to identify the gas, he. For example, by studying emission spectra of the stars, we can determine their chemical composition. A scientist has a sample of an unknown gas. Emission and absorption spectra form the basis of spectroscopy, which uses spectra. Emission Spectra Example.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Example For example, by studying emission spectra of the stars, we can determine their chemical composition. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. This spectrum of radiation emitted by electrons in the excited atoms. Emission Spectra Example.
From www.youtube.com
2.3.2 Distinguish between a continuous spectrum and a line spectrum Emission Spectra Example A scientist has a sample of an unknown gas. In order to identify the gas, he. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The electromagnetic spectrum was divided into regions, many. Emission Spectra Example.
From chemwiki.ucdavis.edu
Light, Particles, and Waves Chemwiki Emission Spectra Example Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Identifying an unknown gas using emission spectra. A scientist has a sample of an unknown gas. In order to identify the gas, he. The. Emission Spectra Example.