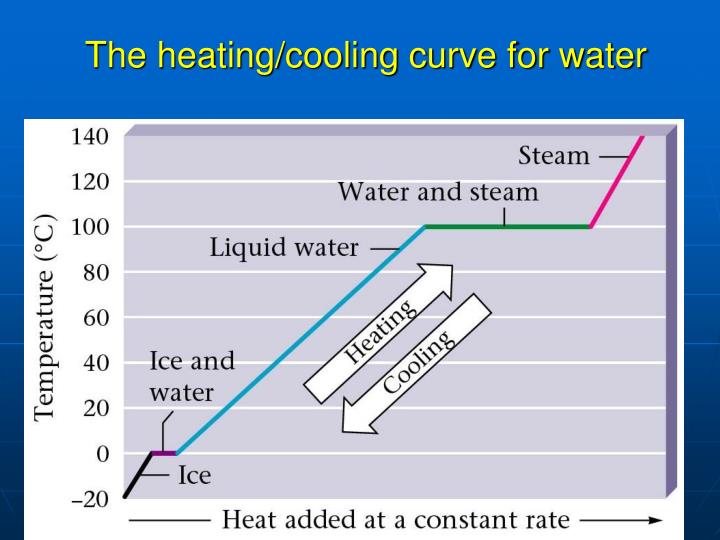
Heating Curve Of Water Experiment Method . Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The sample is initially ice at 1 atm and −23°c; Start each with water at the same temperature and record information from. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. Use the heating curve to explain why the temperature did not increase during state changes. As heat is added, the temperature of the ice increases linearly with time. Practice the experiment before you. Compare cooling curves for beakers with different insulation, lids etc. The experiment described above can be summarized in a graph called a heating curve (figure below).
from lessonstone.z13.web.core.windows.net
Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. As heat is added, the temperature of the ice increases linearly with time. The sample is initially ice at 1 atm and −23°c; Start each with water at the same temperature and record information from. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. Practice the experiment before you. Compare cooling curves for beakers with different insulation, lids etc. Use the heating curve to explain why the temperature did not increase during state changes. The experiment described above can be summarized in a graph called a heating curve (figure below). Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\).
Heating Curve Of Water
Heating Curve Of Water Experiment Method Compare cooling curves for beakers with different insulation, lids etc. Use the heating curve to explain why the temperature did not increase during state changes. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. The sample is initially ice at 1 atm and −23°c; Compare cooling curves for beakers with different insulation, lids etc. Practice the experiment before you. Start each with water at the same temperature and record information from. As heat is added, the temperature of the ice increases linearly with time. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The experiment described above can be summarized in a graph called a heating curve (figure below). Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water.
From wt.kimiq.com
Heating Curve Of Water Water Ionizer Heating Curve Of Water Experiment Method Use the heating curve to explain why the temperature did not increase during state changes. Start each with water at the same temperature and record information from. Practice the experiment before you. The experiment described above can be summarized in a graph called a heating curve (figure below). The sample is initially ice at 1 atm and −23°c; Heat from. Heating Curve Of Water Experiment Method.
From chem.libretexts.org
12.7 Heating Curve for Water Chemistry LibreTexts Heating Curve Of Water Experiment Method Start each with water at the same temperature and record information from. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Use the heating curve to explain why the temperature did not increase during state changes. The experiment described above can be summarized in a graph called a heating. Heating Curve Of Water Experiment Method.
From www.youtube.com
Heating Curve of Water Lab Time Lapse YouTube Heating Curve Of Water Experiment Method Start each with water at the same temperature and record information from. The sample is initially ice at 1 atm and −23°c; Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The experiment described above can be summarized in a graph called a heating. Heating Curve Of Water Experiment Method.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Heating Curve Of Water Experiment Method Start each with water at the same temperature and record information from. Use the heating curve to explain why the temperature did not increase during state changes. As heat is added, the temperature of the ice increases linearly with time. Compare cooling curves for beakers with different insulation, lids etc. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature. Heating Curve Of Water Experiment Method.
From www.slideserve.com
PPT Heating Curves and Thermodynamics PowerPoint Presentation, free Heating Curve Of Water Experiment Method Start each with water at the same temperature and record information from. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Practice the experiment before you. The experiment described above can be summarized in a graph called a heating curve (figure below). The sample. Heating Curve Of Water Experiment Method.
From lessonstone.z13.web.core.windows.net
Heating Curve Of Water Heating Curve Of Water Experiment Method As heat is added, the temperature of the ice increases linearly with time. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. Compare cooling curves for beakers with different insulation, lids etc. Practice the experiment before you. Figure \(\pageindex{3}\) shows a heating curve, a. Heating Curve Of Water Experiment Method.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint Heating Curve Of Water Experiment Method Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Compare cooling curves for beakers with different insulation, lids etc. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Heat from the environment is. Heating Curve Of Water Experiment Method.
From www.youtube.com
Specific Heat Capacity of Water Physics Experiment YouTube Heating Curve Of Water Experiment Method Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The sample is initially ice at 1 atm and −23°c; The experiment described above can. Heating Curve Of Water Experiment Method.
From www.youtube.com
Heating Curve of Water YouTube Heating Curve Of Water Experiment Method Compare cooling curves for beakers with different insulation, lids etc. Use the heating curve to explain why the temperature did not increase during state changes. As heat is added, the temperature of the ice increases linearly with time. The experiment described above can be summarized in a graph called a heating curve (figure below). Once all the liquid has completely. Heating Curve Of Water Experiment Method.
From www.researchgate.net
Heating curve for water. Download Scientific Diagram Heating Curve Of Water Experiment Method The sample is initially ice at 1 atm and −23°c; Use the heating curve to explain why the temperature did not increase during state changes. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Practice the experiment before you. Once all the liquid has completely boiled away, continued heating. Heating Curve Of Water Experiment Method.
From quizlet.com
Heating Curve for Water Diagram Quizlet Heating Curve Of Water Experiment Method The experiment described above can be summarized in a graph called a heating curve (figure below). The sample is initially ice at 1 atm and −23°c; As heat is added, the temperature of the ice increases linearly with time. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its. Heating Curve Of Water Experiment Method.
From chem-net.blogspot.com
Phase Changes Energy Changes Heating Curves Chemistry Net Heating Curve Of Water Experiment Method The sample is initially ice at 1 atm and −23°c; The experiment described above can be summarized in a graph called a heating curve (figure below). Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. Practice the experiment before you. Compare cooling curves for. Heating Curve Of Water Experiment Method.
From quizzlistreplevies.z13.web.core.windows.net
Heating Curve Of Water Diagram Heating Curve Of Water Experiment Method The sample is initially ice at 1 atm and −23°c; Practice the experiment before you. Use the heating curve to explain why the temperature did not increase during state changes. Start each with water at the same temperature and record information from. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in. Heating Curve Of Water Experiment Method.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Of Water Experiment Method Use the heating curve to explain why the temperature did not increase during state changes. Practice the experiment before you. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The experiment described above can be summarized in a graph called a heating curve (figure. Heating Curve Of Water Experiment Method.
From www.slideserve.com
PPT Heating and Cooling Curves of Water PowerPoint Presentation ID Heating Curve Of Water Experiment Method Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Compare cooling curves for beakers with different insulation, lids etc. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The sample is initially ice. Heating Curve Of Water Experiment Method.
From studylib.net
Heating Curve of Water Heating Curve Of Water Experiment Method Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. The sample is initially ice at 1 atm and −23°c; Start each with water at the same temperature and record information from. Compare cooling curves for beakers with different insulation, lids etc. Use the heating. Heating Curve Of Water Experiment Method.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Curve Of Water Experiment Method The sample is initially ice at 1 atm and −23°c; Compare cooling curves for beakers with different insulation, lids etc. The experiment described above can be summarized in a graph called a heating curve (figure below). Practice the experiment before you. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils. Heating Curve Of Water Experiment Method.
From www.youtube.com
Heating Curve and Cooling Curve of Water Enthalpy of Fusion Heating Curve Of Water Experiment Method As heat is added, the temperature of the ice increases linearly with time. The experiment described above can be summarized in a graph called a heating curve (figure below). Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. Practice the experiment before you. Start. Heating Curve Of Water Experiment Method.
From studylib.net
Experiment Heating Curve for Water Heating Curve Of Water Experiment Method Practice the experiment before you. Use the heating curve to explain why the temperature did not increase during state changes. Start each with water at the same temperature and record information from. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. The sample is. Heating Curve Of Water Experiment Method.
From www.researchgate.net
Heating curve of water at fixed atmospheric pressure. It corresponds to Heating Curve Of Water Experiment Method Practice the experiment before you. The experiment described above can be summarized in a graph called a heating curve (figure below). As heat is added, the temperature of the ice increases linearly with time. Use the heating curve to explain why the temperature did not increase during state changes. Heat from the environment is used to vaporize the refrigerant, which. Heating Curve Of Water Experiment Method.
From screenpal.com
Heating Curve of Water in Real Time Heating Curve Of Water Experiment Method Use the heating curve to explain why the temperature did not increase during state changes. Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The sample is initially ice at 1 atm and −23°c; The experiment described above can be summarized in a graph. Heating Curve Of Water Experiment Method.
From www.youtube.com
Heating Curve of Water Time lapse with Graph YouTube Heating Curve Of Water Experiment Method Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Compare cooling curves for beakers with different insulation, lids etc. Use the heating curve to explain why the temperature did not increase during state changes. Start each with water at the same temperature and record information from. Once all the. Heating Curve Of Water Experiment Method.
From www.youtube.com
Gr 10 Physical Sciences Experiment Labs Cooling and Heating Curve Heating Curve Of Water Experiment Method The sample is initially ice at 1 atm and −23°c; Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The experiment described above can. Heating Curve Of Water Experiment Method.
From www.docsity.com
THE HEATING CURVE OF WATER Slides Chemistry Docsity Heating Curve Of Water Experiment Method Compare cooling curves for beakers with different insulation, lids etc. Use the heating curve to explain why the temperature did not increase during state changes. Practice the experiment before you. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. As heat is added, the temperature of the ice increases. Heating Curve Of Water Experiment Method.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Curve Of Water Experiment Method The experiment described above can be summarized in a graph called a heating curve (figure below). Practice the experiment before you. Compare cooling curves for beakers with different insulation, lids etc. As heat is added, the temperature of the ice increases linearly with time. Once all the liquid has completely boiled away, continued heating of the steam (since the container. Heating Curve Of Water Experiment Method.
From studylib.net
Heating Curve of Water Lab (1) Heating Curve Of Water Experiment Method Compare cooling curves for beakers with different insulation, lids etc. Start each with water at the same temperature and record information from. The experiment described above can be summarized in a graph called a heating curve (figure below). Use the heating curve to explain why the temperature did not increase during state changes. Figure \(\pageindex{3}\) shows a heating curve, a. Heating Curve Of Water Experiment Method.
From studylib.net
Heating Curve of Water Heating Curve Of Water Experiment Method Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. Use the heating curve to explain why the temperature did not increase during state changes.. Heating Curve Of Water Experiment Method.
From www.youtube.com
Heating Curve of Water YouTube Heating Curve Of Water Experiment Method Practice the experiment before you. The experiment described above can be summarized in a graph called a heating curve (figure below). Use the heating curve to explain why the temperature did not increase during state changes. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide. Heating Curve Of Water Experiment Method.
From ch302.cm.utexas.edu
heating curve Heating Curve Of Water Experiment Method Use the heating curve to explain why the temperature did not increase during state changes. Start each with water at the same temperature and record information from. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. Compare cooling curves for beakers with different insulation,. Heating Curve Of Water Experiment Method.
From ar.inspiredpencil.com
Graph Heating Curve For Water Heating Curve Of Water Experiment Method Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Practice the experiment before you. Compare cooling curves for beakers with different insulation, lids etc. Start each with water at the same temperature and record information from. As heat is added, the temperature of the. Heating Curve Of Water Experiment Method.
From studylib.net
IB1 Physics Heating Curve of Water Lab Heating Curve Of Water Experiment Method Practice the experiment before you. The sample is initially ice at 1 atm and −23°c; Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). Compare cooling curves for beakers with different insulation, lids etc. As heat is added, the temperature of the ice increases. Heating Curve Of Water Experiment Method.
From brainly.com
Examine the heating curve for water below. Answer each question Heating Curve Of Water Experiment Method Once all the liquid has completely boiled away, continued heating of the steam (since the container is closed) will increase its temperature above \(100^\text{o} \text{c}\). The sample is initially ice at 1 atm and −23°c; Practice the experiment before you. Use the heating curve to explain why the temperature did not increase during state changes. Start each with water at. Heating Curve Of Water Experiment Method.
From studylib.net
Heating Curve Lab Heating Curve Of Water Experiment Method Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Compare cooling curves for beakers with different insulation, lids etc. As heat is added, the temperature of the ice increases linearly with time. Start each with water at the same temperature and record information from. Once all the liquid has. Heating Curve Of Water Experiment Method.
From heatingwaterdzumekuchi.blogspot.com
Heating Water Heating Water Experiment Heating Curve Of Water Experiment Method Use the heating curve to explain why the temperature did not increase during state changes. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. Compare cooling curves for beakers with different insulation, lids etc. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature. Heating Curve Of Water Experiment Method.
From studylib.net
Lab Heating Curve of Water Heating Curve Of Water Experiment Method Practice the experiment before you. As heat is added, the temperature of the ice increases linearly with time. Heat from the environment is used to vaporize the refrigerant, which is then condensed to a liquid in coils within a house to provide heat. Start each with water at the same temperature and record information from. Figure \(\pageindex{3}\) shows a heating. Heating Curve Of Water Experiment Method.