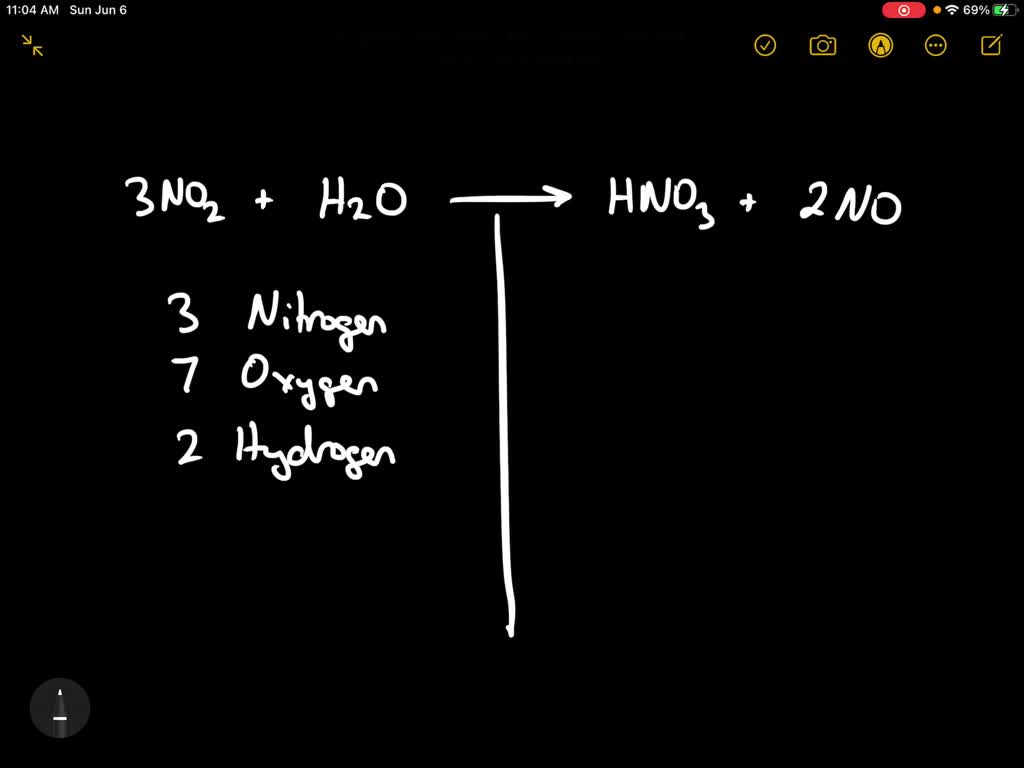
Iron Ii Oxide Nitric Acid Balanced Equation . To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A more complex redox reaction occurs when copper dissolves in nitric acid. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. The balanced equation will appear. The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. Enter an equation of an ionic chemical equation and press the balance button. Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. The acid attacks the metal vigorously, and large quantities of the. The balanced equation will be calculated along with the.
from www.numerade.com
The acid attacks the metal vigorously, and large quantities of the. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. A more complex redox reaction occurs when copper dissolves in nitric acid. Enter an equation of an ionic chemical equation and press the balance button. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). The balanced equation will appear. The balanced equation will be calculated along with the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient.
SOLVEDIs the following chemical equation balanced? This reaction is
Iron Ii Oxide Nitric Acid Balanced Equation A more complex redox reaction occurs when copper dissolves in nitric acid. Enter an equation of an ionic chemical equation and press the balance button. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. The balanced equation will be calculated along with the. The acid attacks the metal vigorously, and large quantities of the. Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. A more complex redox reaction occurs when copper dissolves in nitric acid.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Iron Ii Oxide Nitric Acid Balanced Equation The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.youtube.com
How to write the formula for iron (II) oxide YouTube Iron Ii Oxide Nitric Acid Balanced Equation Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. Enter an equation of an ionic chemical equation and press the balance button. Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balancing chemical equations write a balanced equation for. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced molecular equation and a net ionic equation for Iron Ii Oxide Nitric Acid Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). The acid attacks the metal vigorously, and large quantities of the. Enter. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.chegg.com
Solved 5. Iron II oxide reacts violently and exothermically Iron Ii Oxide Nitric Acid Balanced Equation Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. The balanced equation will be calculated along with the. A more complex redox reaction occurs when copper dissolves in nitric acid. The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVED Aluminum metal reacts with aqueous iron(II) oxide to form Iron Ii Oxide Nitric Acid Balanced Equation Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. The acid attacks the metal vigorously, and large quantities of the. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. Balancing. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.toppr.com
Illustrate how a metal like copper can give different products with Iron Ii Oxide Nitric Acid Balanced Equation The balanced equation will be calculated along with the. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. The balanced equation will appear. Enter an equation of an ionic chemical equation and press the balance button. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. The acid attacks the metal. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Iron Ii Oxide Nitric Acid Balanced Equation The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A more complex redox reaction occurs when copper dissolves in nitric acid. Balancing chemical equations write a. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
Nitric acid is a component of acid rain that forms when gaseous Iron Ii Oxide Nitric Acid Balanced Equation We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.chegg.com
Solved Write a balanced equation for the reaction of nitric Iron Ii Oxide Nitric Acid Balanced Equation The acid attacks the metal vigorously, and large quantities of the. The balanced equation will be calculated along with the. The balanced equation will appear. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. Enter an equation of an ionic chemical equation and press the balance button. To balance a chemical equation, enter an equation of a chemical reaction and press the balance. Iron Ii Oxide Nitric Acid Balanced Equation.
From slideplayer.com
Mixing Acids and Bases. ppt download Iron Ii Oxide Nitric Acid Balanced Equation The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). The acid attacks the metal vigorously, and large quantities of the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A more complex redox reaction occurs. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced net ionic equation for each of the following Iron Ii Oxide Nitric Acid Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. The balanced equation will appear. The balanced equation will be calculated along with the. A more complex redox reaction occurs when copper dissolves in nitric acid. The acid attacks the metal. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVEDSome metals, such as iron, can be oxidized to more than one Iron Ii Oxide Nitric Acid Balanced Equation The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. A more complex redox reaction occurs when copper dissolves in nitric acid. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a.. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVED Solid iron(II) hydroxide to form solid iron(II Iron Ii Oxide Nitric Acid Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. A more complex redox reaction occurs when copper dissolves in nitric acid. The balanced equation will be. Iron Ii Oxide Nitric Acid Balanced Equation.
From testbook.com
Iron (II) Oxide Formula Structure, Preparation & Properties Iron Ii Oxide Nitric Acid Balanced Equation Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Enter an equation of an ionic chemical equation and press the balance button. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. The balanced. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.youtube.com
How to Balance FeSO4 = Fe2O3 + SO2 + SO3 of Iron (II Iron Ii Oxide Nitric Acid Balanced Equation The balanced equation will be calculated along with the. The acid attacks the metal vigorously, and large quantities of the. Enter an equation of an ionic chemical equation and press the balance button. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. A more complex redox reaction. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Iron Ii Oxide Nitric Acid Balanced Equation The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). The balanced equation will appear. Enter an equation of an ionic chemical equation and press the balance button. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. To balance a chemical equation, enter an equation of a. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the Iron Ii Oxide Nitric Acid Balanced Equation The balanced equation will be calculated along with the. Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. Enter an equation of an ionic chemical equation and press the balance button. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must. Iron Ii Oxide Nitric Acid Balanced Equation.
From meryes.weebly.com
Balanced chemical equation with state symbols calculator meryes Iron Ii Oxide Nitric Acid Balanced Equation The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. To balance a chemical equation, enter an equation. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVEDSome metals, such as thallium, can be oxidized to more than one Iron Ii Oxide Nitric Acid Balanced Equation The balanced equation will be calculated along with the. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The acid. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.tes.com
Ionic Equations Acids and Salts Edexcel 91 Combined Science Teaching Iron Ii Oxide Nitric Acid Balanced Equation The balanced equation will appear. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. The acid attacks the metal vigorously, and. Iron Ii Oxide Nitric Acid Balanced Equation.
From pubs.acs.org
Enhancement of Iron(II)Dependent Reduction of Nitrite to Nitric Oxide Iron Ii Oxide Nitric Acid Balanced Equation The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. The reaction of elemental zinc with arsenic acid in acidic. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.pw.live
Iron II Oxide Formula Structure, Properties, Uses Iron Ii Oxide Nitric Acid Balanced Equation Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. The balanced equation will appear. The acid attacks the metal vigorously, and large quantities of the. The balanced equation will be calculated along with the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A more complex redox reaction occurs when copper dissolves in nitric acid.. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.chegg.com
Solved Iron solid can react with dilute nitric acid to form Iron Ii Oxide Nitric Acid Balanced Equation Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. A more complex redox reaction occurs when copper dissolves in nitric acid. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. The balanced equation will. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVED Write balanced chemical equations for the reaction between iron Iron Ii Oxide Nitric Acid Balanced Equation The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). The balanced equation will appear. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. Enter an equation of an ionic chemical equation and press the balance button. Balancing chemical equations write a balanced equation for the reaction. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.slideshare.net
Acids And Bases Iron Ii Oxide Nitric Acid Balanced Equation The balanced equation will appear. The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. Iron (ii) oxide reacts with nitric acid. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVED 'In this laboratory experiment; two oxides of iron can form Iron Ii Oxide Nitric Acid Balanced Equation The balanced equation will appear. The acid attacks the metal vigorously, and large quantities of the. Enter an equation of an ionic chemical equation and press the balance button. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVED 369 of RedoxBalancing Which of the following is the correctly Iron Ii Oxide Nitric Acid Balanced Equation The acid attacks the metal vigorously, and large quantities of the. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. A more complex redox reaction occurs when copper dissolves in nitric acid. Iron (ii) oxide reacts with nitric acid to form the iron. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVEDMarine Chemistry of Iron On the seafloor, solid iron(II) oxide Iron Ii Oxide Nitric Acid Balanced Equation Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. The balanced equation will appear. The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and zn 2 + (aq). Enter an equation of an ionic chemical. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.slideserve.com
PPT Unit 6 Chemical Reactions PowerPoint Presentation, free download Iron Ii Oxide Nitric Acid Balanced Equation Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. The acid attacks the metal vigorously, and large quantities of the. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. The balanced equation will be calculated along with the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVEDWrite complete balanced halfreactions for (a) reduction of Iron Ii Oxide Nitric Acid Balanced Equation The acid attacks the metal vigorously, and large quantities of the. The balanced equation will appear. A more complex redox reaction occurs when copper dissolves in nitric acid. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. Feo (s)+hno3 (aq)→fe+3 (aq)+hno2 (aq) a. The reaction of elemental. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVEDIs the following chemical equation balanced? This reaction is Iron Ii Oxide Nitric Acid Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. The balanced equation will appear. Balancing chemical equations write a balanced equation. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.youtube.com
How to Write the Formula for Iron (II) oxide YouTube Iron Ii Oxide Nitric Acid Balanced Equation The acid attacks the metal vigorously, and large quantities of the. The balanced equation will be calculated along with the. The balanced equation will appear. Enter an equation of an ionic chemical equation and press the balance button. The reaction of elemental zinc with arsenic acid in acidic solution yields arsine (ash 3, a highly toxic and unstable gas) and. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron Ii Oxide Nitric Acid Balanced Equation Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. The acid attacks the metal vigorously, and large quantities of the. A more complex redox reaction occurs when copper dissolves in nitric. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.essentialchemicalindustry.org
Nitric acid Iron Ii Oxide Nitric Acid Balanced Equation We can balance the least complex substance, o 2, but because there are 2 oxygen atoms per o 2 molecule, we must use a fractional coefficient. The balanced equation will appear. Iron (ii) oxide reacts with nitric acid to form the iron (iii) cation and nitrous acid. To balance a chemical equation, enter an equation of a chemical reaction and. Iron Ii Oxide Nitric Acid Balanced Equation.
From www.numerade.com
SOLVED(i) Calculate oxidation number of (a) Fe in FezO3 (b) Pin KzHPO4 Iron Ii Oxide Nitric Acid Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Balancing chemical equations write a balanced equation for the reaction of molecular nitrogen (n 2) and oxygen (o 2) to form dinitrogen. A more complex redox reaction occurs when copper dissolves in nitric acid. The balanced equation will appear. The balanced equation will be calculated along with. Iron Ii Oxide Nitric Acid Balanced Equation.