Anodes And Cathodes Explained . an anode is an electrode by which the conventional current enters into a polarized electrical device. Anodes are negative electrodes that release electrons. the anode and cathode. One electrode is positive, whilst the other is negative. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. cathodes are positive electrodes that receive electrons. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. Inside of a battery, anodes and.
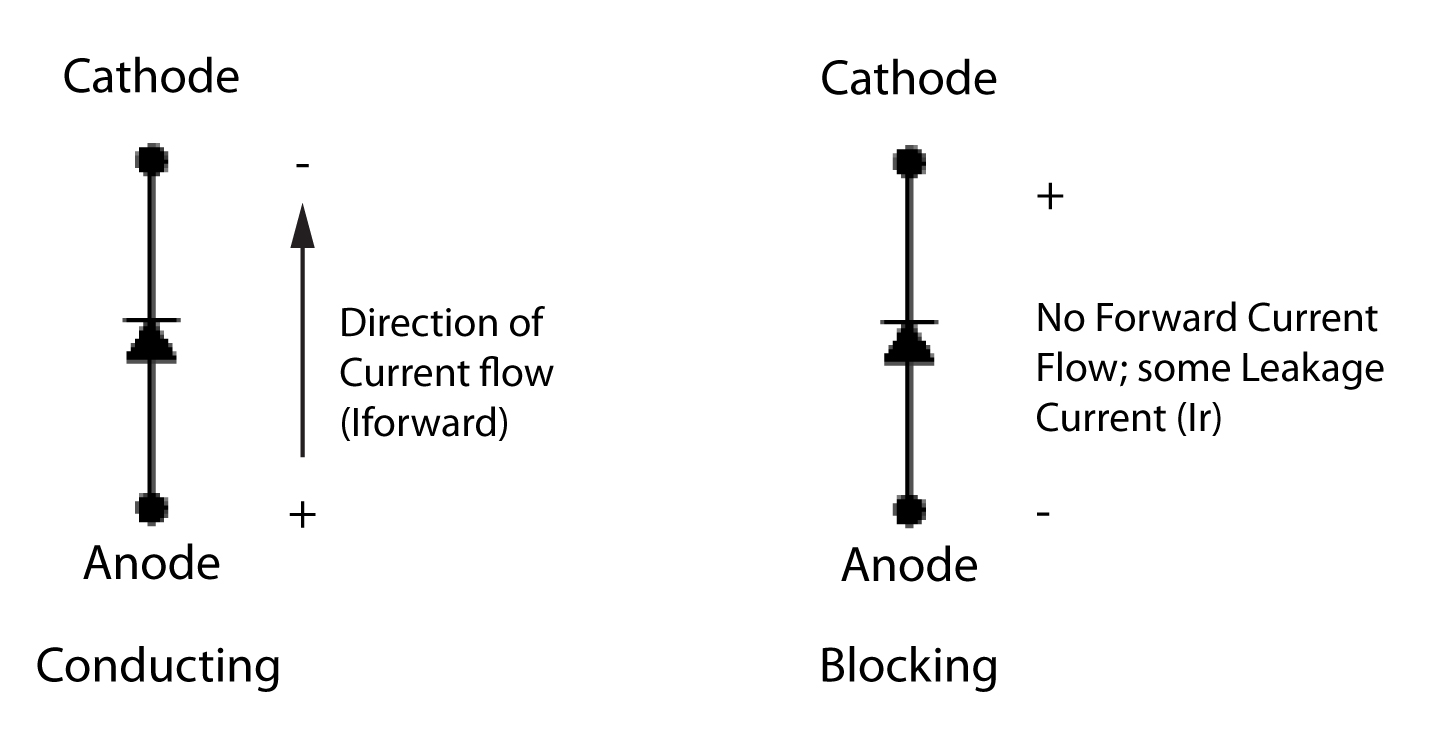
from voltagemultipliers.blogspot.ca
One electrode is positive, whilst the other is negative. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. cathodes are positive electrodes that receive electrons. Inside of a battery, anodes and. the anode and cathode. an anode is an electrode by which the conventional current enters into a polarized electrical device. Anodes are negative electrodes that release electrons. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode.
Voltage Multipliers Inc. Anode vs. Cathode in a High Voltage Diode
Anodes And Cathodes Explained an anode is an electrode by which the conventional current enters into a polarized electrical device. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. an anode is an electrode by which the conventional current enters into a polarized electrical device. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. the anode and cathode. Anodes are negative electrodes that release electrons. cathodes are positive electrodes that receive electrons. One electrode is positive, whilst the other is negative. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. Inside of a battery, anodes and. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Anodes And Cathodes Explained Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. Anodes are negative electrodes that release electrons. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. the anode and cathode. cathodes are positive electrodes that receive electrons. learn. Anodes And Cathodes Explained.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode Anodes And Cathodes Explained learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. Conversely, the cathode facilitates reduction and serves as the positive electrode in. Anodes And Cathodes Explained.
From www.dreamstime.com
Anode And Cathode Scientific Physics Education Diagram, Vector Anodes And Cathodes Explained One electrode is positive, whilst the other is negative. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. learn what cathode and. Anodes And Cathodes Explained.
From www.differencebetween.com
Difference Between Anode and Cathode Compare the Difference Between Anodes And Cathodes Explained the anode and cathode. One electrode is positive, whilst the other is negative. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. Inside of a battery, anodes and. an anode is an electrode by which the conventional current enters into a polarized electrical device. cathodes are positive. Anodes And Cathodes Explained.
From www.researchgate.net
3 Arrangement of Anodes and Cathodes in soil Anodes And Cathodes Explained Anodes are negative electrodes that release electrons. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. Inside of a battery, anodes and. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. an anode. Anodes And Cathodes Explained.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Anodes And Cathodes Explained Anodes are negative electrodes that release electrons. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. cathodes are positive electrodes that receive electrons. an anode is an electrode by which the conventional current enters into a polarized electrical device. the anode and cathode.. Anodes And Cathodes Explained.
From www.thoughtco.com
How to Define Anode and Cathode Anodes And Cathodes Explained in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. One electrode is positive, whilst the other is negative. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you. Anodes And Cathodes Explained.
From www.researchgate.net
A summary of the various types of cathodes, anodes and electrolytes Anodes And Cathodes Explained Anodes are negative electrodes that release electrons. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. One electrode is positive, whilst the other is negative. Inside of. Anodes And Cathodes Explained.
From jadafinhawkins.blogspot.com
Anode and Cathode in Electrolysis JadafinHawkins Anodes And Cathodes Explained One electrode is positive, whilst the other is negative. Inside of a battery, anodes and. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. cathodes are positive electrodes that receive. Anodes And Cathodes Explained.
From www.researchgate.net
Summary of anodes, cathodes, and solidstate electrolytes synthesized Anodes And Cathodes Explained One electrode is positive, whilst the other is negative. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. an anode is an electrode by which the conventional current enters into a polarized electrical device. the anode and cathode. cathodes are positive electrodes that. Anodes And Cathodes Explained.
From mavink.com
Anode Cathode Diagram Anodes And Cathodes Explained learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. an anode is an electrode by which the conventional current enters into a polarized electrical device. One electrode is positive, whilst the other is negative. the anode and cathode. Conversely, the cathode facilitates reduction and serves as the positive. Anodes And Cathodes Explained.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Anodes And Cathodes Explained Inside of a battery, anodes and. Anodes are negative electrodes that release electrons. the anode and cathode. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. in a galvanic cell, the anode undergoes oxidation and functions as the. Anodes And Cathodes Explained.
From fixlibrarycipponiih.z13.web.core.windows.net
What Happens At The Cathode In Electrolysis Anodes And Cathodes Explained cathodes are positive electrodes that receive electrons. the anode and cathode. One electrode is positive, whilst the other is negative. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. Inside of a battery,. Anodes And Cathodes Explained.
From www.slideserve.com
PPT Alessandro Volta PowerPoint Presentation ID1614332 Anodes And Cathodes Explained In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction. Anodes And Cathodes Explained.
From askanydifference.com
Cathode vs Anode Difference and Comparison Anodes And Cathodes Explained Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. an anode is an electrode by which the conventional current enters into a polarized electrical device. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. Anodes. Anodes And Cathodes Explained.
From studybreathings.z21.web.core.windows.net
Do Electrons Flow From Anode To Cathode Anodes And Cathodes Explained in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. One electrode is positive, whilst the other is negative. cathodes are positive electrodes that receive electrons. learn what cathode and. Anodes And Cathodes Explained.
From www.researchgate.net
12 Busbars in the package, with location of anodes and cathodes Anodes And Cathodes Explained learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. cathodes are positive electrodes that receive electrons. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as. Anodes And Cathodes Explained.
From www.slideserve.com
PPT m > e g Drift Chambers PowerPoint Presentation, free download Anodes And Cathodes Explained cathodes are positive electrodes that receive electrons. Anodes are negative electrodes that release electrons. an anode is an electrode by which the conventional current enters into a polarized electrical device. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. One electrode is positive, whilst. Anodes And Cathodes Explained.
From www.reddit.com
Anode vs Cathode clarifications r/Mcat Anodes And Cathodes Explained Inside of a battery, anodes and. an anode is an electrode by which the conventional current enters into a polarized electrical device. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. Anodes are negative electrodes that release electrons. the anode and cathode. cathodes. Anodes And Cathodes Explained.
From batteryfast.com
Anode Vs Cathode BatteryFast Anodes And Cathodes Explained In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive. Anodes And Cathodes Explained.
From www.youtube.com
How to identify ANODE and CATHODE in LED YouTube Anodes And Cathodes Explained the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. the anode and cathode. cathodes are. Anodes And Cathodes Explained.
From www.collegesearch.in
Cathode and Anode Definition, Examples, Differences CollegeSearch Anodes And Cathodes Explained Anodes are negative electrodes that release electrons. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. One electrode is positive, whilst the other. Anodes And Cathodes Explained.
From www.batterypowertips.com
What is a cathode? Battery Power Tips Anodes And Cathodes Explained cathodes are positive electrodes that receive electrons. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. the anode and cathode. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. an anode. Anodes And Cathodes Explained.
From forumautomation.com
6 Differences between Anode and Cathode Electronics Industrial Anodes And Cathodes Explained In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. Inside of a battery, anodes and. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. the two compartments of an electrochemical cell where the half reactions occur are called the. Anodes And Cathodes Explained.
From www.researchgate.net
The principle of the lithiumion battery (LiB) showing the Anodes And Cathodes Explained Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. the anode and cathode. Inside of a battery, anodes and. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. an anode is an electrode by. Anodes And Cathodes Explained.
From www.slideserve.com
PPT Electrochemical Engineering & Alternate Energy PowerPoint Anodes And Cathodes Explained Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. the anode and cathode. Inside of a battery, anodes and. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. learn what cathode and anode are in electrochemistry, how they. Anodes And Cathodes Explained.
From study.com
Anode vs. Cathode in Electrochemical Cells Reaction & Notation Anodes And Cathodes Explained the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. One electrode is positive, whilst the other is negative. Inside of a battery, anodes and. an anode is an electrode by which the conventional current enters into a polarized electrical. Anodes And Cathodes Explained.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube Anodes And Cathodes Explained an anode is an electrode by which the conventional current enters into a polarized electrical device. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. Inside of a battery, anodes and. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive. Anodes And Cathodes Explained.
From voltagemultipliers.blogspot.ca
Voltage Multipliers Inc. Anode vs. Cathode in a High Voltage Diode Anodes And Cathodes Explained an anode is an electrode by which the conventional current enters into a polarized electrical device. the anode and cathode. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. in a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode.. Anodes And Cathodes Explained.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation ID2216847 Anodes And Cathodes Explained Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. Inside of a battery, anodes and. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. in. Anodes And Cathodes Explained.
From mytewifi.weebly.com
Cathode anode diagram mytewifi Anodes And Cathodes Explained the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. in a galvanic cell, the anode undergoes oxidation and functions as. Anodes And Cathodes Explained.
From dragonflyenergy.com
Anode vs Cathode What's the Difference? Dragonfly Energy Anodes And Cathodes Explained Inside of a battery, anodes and. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. One electrode is positive, whilst the other is negative. cathodes are positive electrodes that receive electrons. Anodes are negative electrodes that release electrons. an anode is an electrode by which the conventional current. Anodes And Cathodes Explained.
From www.youtube.com
Three methods of finding Anode and Cathode of the LED (Light Emitting Anodes And Cathodes Explained Inside of a battery, anodes and. cathodes are positive electrodes that receive electrons. the anode and cathode. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. One electrode is positive, whilst the other is negative. Anodes are negative electrodes that release electrons. learn. Anodes And Cathodes Explained.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5744606 Anodes And Cathodes Explained cathodes are positive electrodes that receive electrons. learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. an anode is an electrode by which the conventional current enters into a polarized electrical device. In contrasts with a cathode, an electrode by which conventional current leaves an electrical device. . Anodes And Cathodes Explained.
From www.aquametals.com
What Are Battery Anode and Cathode Materials? AquaMetals Anodes And Cathodes Explained learn what cathode and anode are in electrochemistry, how they differ in polarization and reaction type, and how. the anode and cathode. Anodes are negative electrodes that release electrons. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an electrode that you can.. Anodes And Cathodes Explained.