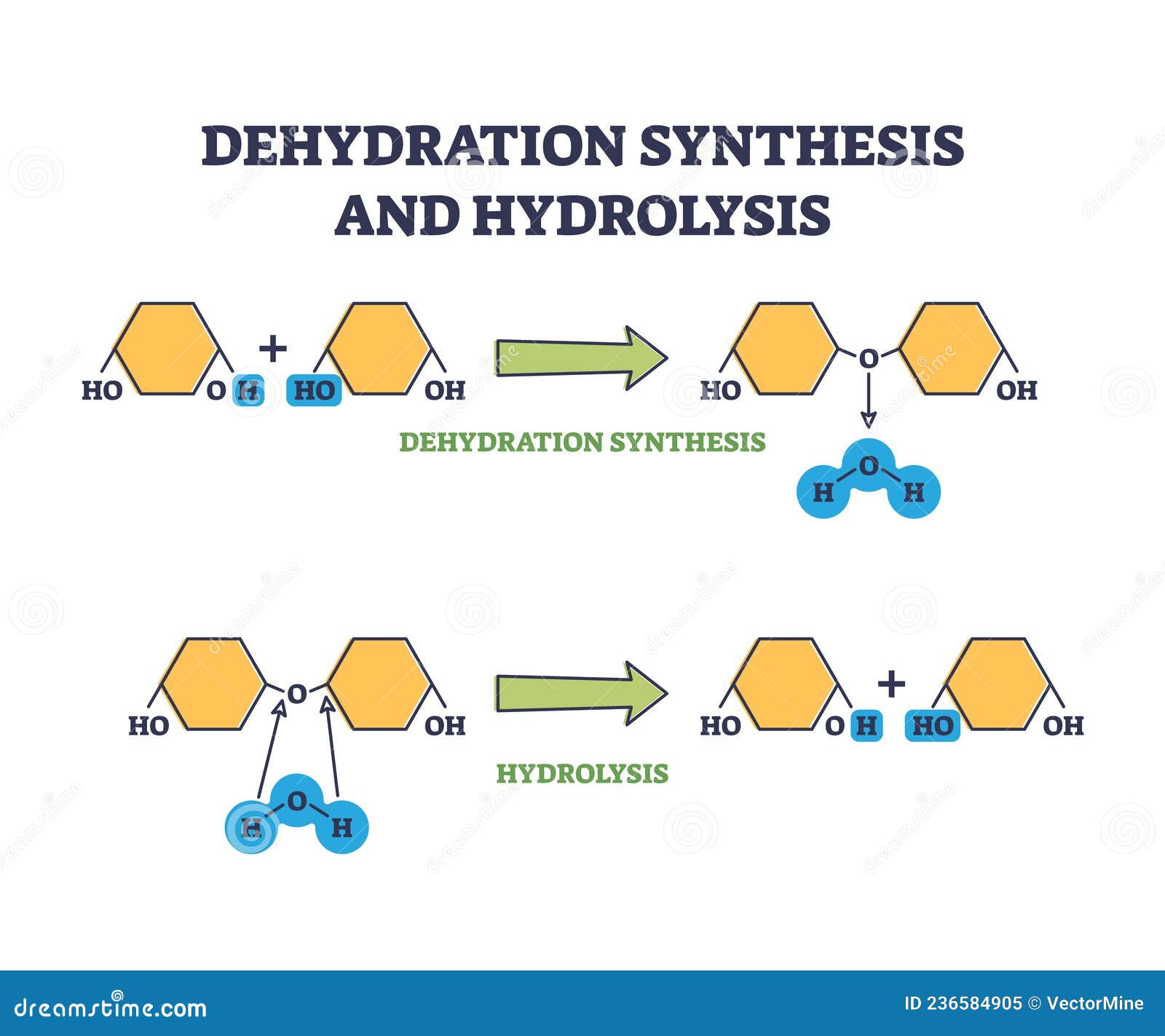
What Do Hydrolysis Reactions Do . Let’s take a close look at the mechanism for hydrolysis. A salt is formed between the reaction of an acid and a base. And typically, water is used to break chemical bonds in the other reactant. Hydrolysis is a type of decomposition reaction where one of the reactants is water; a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Usually, a neutral salt is formed when a. hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with each other, producing water as one of the products. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance.
from www.dreamstime.com
Hydrolysis is a type of decomposition reaction where one of the reactants is water; Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. Let’s take a close look at the mechanism for hydrolysis. A salt is formed between the reaction of an acid and a base. Usually, a neutral salt is formed when a. Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with each other, producing water as one of the products. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. And typically, water is used to break chemical bonds in the other reactant. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules.
Dehydration Synthesis and Hydrolysis Chemical Process Stages Outline
What Do Hydrolysis Reactions Do the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Let’s take a close look at the mechanism for hydrolysis. Usually, a neutral salt is formed when a. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. A salt is formed between the reaction of an acid and a base. And typically, water is used to break chemical bonds in the other reactant. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Hydrolysis is a type of decomposition reaction where one of the reactants is water; in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with each other, producing water as one of the products.
From mungfali.com
Hydrolysis Reaction Example What Do Hydrolysis Reactions Do Let’s take a close look at the mechanism for hydrolysis. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. And typically, water is. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID307111 What Do Hydrolysis Reactions Do Usually, a neutral salt is formed when a. hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. a hydrolysis reaction is a reaction in which one molecule. What Do Hydrolysis Reactions Do.
From www.dreamstime.com
Dehydration Synthesis and Hydrolysis Chemical Process Stages Outline What Do Hydrolysis Reactions Do hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. A salt is formed between the reaction of an acid and a base. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. What Do Hydrolysis Reactions Do.
From www.youtube.com
Hydrolysis and Dehydration Synthesis Reactions YouTube What Do Hydrolysis Reactions Do Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with each other, producing water as one of the products. And typically, water is used to break chemical bonds in the other reactant. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a. What Do Hydrolysis Reactions Do.
From www.clutchprep.com
Hydrolysis Organic Chemistry Video Clutch Prep What Do Hydrolysis Reactions Do Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. A salt is formed between the reaction of an acid and a base. Usually, a neutral salt is formed when a. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. hydrolysis, in chemistry and physiology,. What Do Hydrolysis Reactions Do.
From mungfali.com
Hydrolysis Reaction Mechanism What Do Hydrolysis Reactions Do a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Usually, a neutral salt is formed when a. Let’s take a. What Do Hydrolysis Reactions Do.
From hubpages.com
Complete Chemistry of Hydrolysis & Hydration. HubPages What Do Hydrolysis Reactions Do in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. Let’s take a close look at the mechanism for hydrolysis. Hydrolysis is a type of decomposition reaction where one of the. What Do Hydrolysis Reactions Do.
From www.animalia-life.club
Simple Hydrolysis Reaction What Do Hydrolysis Reactions Do hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Hydrolysis is a type of decomposition reaction where. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT Ch 3 Biochemistry PowerPoint Presentation, free download ID2827949 What Do Hydrolysis Reactions Do And typically, water is used to break chemical bonds in the other reactant. Usually, a neutral salt is formed when a. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. the definition of hydrolysis is the breaking of a chemical bond through a reaction. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT Organic Reactions Condensation, Hydrolysis, and Substitution What Do Hydrolysis Reactions Do Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Usually, a neutral salt is formed when a. And typically, water is used. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID143913 What Do Hydrolysis Reactions Do a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Hydrolysis may be considered the reverse of a condensation reaction, in. What Do Hydrolysis Reactions Do.
From mungfali.com
Hydrolysis Reaction Diagram What Do Hydrolysis Reactions Do the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Let’s take a close look. What Do Hydrolysis Reactions Do.
From www.pinterest.com
dehydration synthesis / hydrolysis Chemistry lessons, Chemistry What Do Hydrolysis Reactions Do And typically, water is used to break chemical bonds in the other reactant. A salt is formed between the reaction of an acid and a base. Let’s take a close look at the mechanism for hydrolysis. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance.. What Do Hydrolysis Reactions Do.
From www.animalia-life.club
Simple Hydrolysis Reaction What Do Hydrolysis Reactions Do the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. And typically, water is used to break chemical bonds in the other reactant. Hydrolysis is a type of decomposition reaction where one of the reactants is water; in its simplest definition, hydrolysis is a chemical reaction in which water is used to. What Do Hydrolysis Reactions Do.
From www.animalia-life.club
Simple Hydrolysis Reaction What Do Hydrolysis Reactions Do Let’s take a close look at the mechanism for hydrolysis. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine. What Do Hydrolysis Reactions Do.
From www.youtube.com
salt hydrolysis and net ionic equations review YouTube What Do Hydrolysis Reactions Do hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. A salt is formed between the reaction of an acid and a base. Usually, a neutral salt is formed when a. Hydrolysis is a type of decomposition reaction where one of the reactants is water; a hydrolysis reaction is a reaction in. What Do Hydrolysis Reactions Do.
From www.sciencelearn.org.nz
Hydrolysis reaction — Science Learning Hub What Do Hydrolysis Reactions Do in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. And typically, water is used to break chemical bonds in the other reactant. Usually, a neutral salt is formed when a. hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of. What Do Hydrolysis Reactions Do.
From www.chemistrysteps.com
Amide Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps What Do Hydrolysis Reactions Do Usually, a neutral salt is formed when a. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. And typically,. What Do Hydrolysis Reactions Do.
From www.chemistrysteps.com
Acetal Hydrolysis Mechanism Chemistry Steps What Do Hydrolysis Reactions Do a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Let’s take a close look at the mechanism for hydrolysis. A salt is formed between the reaction of an acid and a base. Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with each other, producing. What Do Hydrolysis Reactions Do.
From www.chemistrysteps.com
The Mechanism of Nitrile Hydrolysis To Carboxylic Acid Chemistry Steps What Do Hydrolysis Reactions Do Hydrolysis is a type of decomposition reaction where one of the reactants is water; the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. And typically, water is used to break chemical bonds in the other reactant. Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with. What Do Hydrolysis Reactions Do.
From www.britannica.com
Hydrolysis Definition, Examples, & Facts Britannica What Do Hydrolysis Reactions Do a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. And typically, water is used to break chemical bonds in the other reactant. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. Hydrolysis is a type of. What Do Hydrolysis Reactions Do.
From www.chemistrysteps.com
Acetal Hydrolysis Mechanism Chemistry Steps What Do Hydrolysis Reactions Do Hydrolysis is a type of decomposition reaction where one of the reactants is water; in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. And typically, water is used to break chemical bonds in the other reactant. a hydrolysis reaction is a reaction in which. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT HYDROLYSIS REACTIONS PowerPoint Presentation, free download ID What Do Hydrolysis Reactions Do Let’s take a close look at the mechanism for hydrolysis. Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with each other, producing water as one of the products. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. A salt is formed between the reaction. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT Chapter 5 The Molecules of Life PowerPoint Presentation, free What Do Hydrolysis Reactions Do Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with each other, producing water as one of the products. And typically, water is used to break chemical bonds in the other reactant. a hydrolysis reaction is a reaction. What Do Hydrolysis Reactions Do.
From www.animalia-life.club
Simple Hydrolysis Reaction What Do Hydrolysis Reactions Do Hydrolysis is a type of decomposition reaction where one of the reactants is water; And typically, water is used to break chemical bonds in the other reactant. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine. What Do Hydrolysis Reactions Do.
From www.researchgate.net
The process of protein hydrolysis and its products. (A) chemical What Do Hydrolysis Reactions Do Let’s take a close look at the mechanism for hydrolysis. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. Usually, a neutral salt is formed when a. Hydrolysis may be considered the reverse of a condensation. What Do Hydrolysis Reactions Do.
From open.oregonstate.education
2.4 Compounds Essential to Human Functioning Anatomy What Do Hydrolysis Reactions Do Usually, a neutral salt is formed when a. hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. the definition of hydrolysis is the breaking of a chemical. What Do Hydrolysis Reactions Do.
From www.scribd.com
Hydrolysis Reactions PDF What Do Hydrolysis Reactions Do And typically, water is used to break chemical bonds in the other reactant. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Let’s take a close look at the mechanism for hydrolysis. hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. in its. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT Chapter 10 Organic Reactions Pathways to New Products What Do Hydrolysis Reactions Do Let’s take a close look at the mechanism for hydrolysis. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with each other, producing water as one of the products. Usually, a neutral salt is formed when a. And typically, water. What Do Hydrolysis Reactions Do.
From www.animalia-life.club
Simple Hydrolysis Reaction What Do Hydrolysis Reactions Do Hydrolysis may be considered the reverse of a condensation reaction, in which two molecules combine with each other, producing water as one of the products. Usually, a neutral salt is formed when a. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. A salt is formed between the reaction of an acid and a. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT HYDROLYSIS REACTIONS PowerPoint Presentation, free download ID What Do Hydrolysis Reactions Do hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break down with water. a hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Hydrolysis may be considered the reverse of a condensation reaction,. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT CHAPTER 5 PowerPoint Presentation, free download ID1473626 What Do Hydrolysis Reactions Do Usually, a neutral salt is formed when a. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Let’s take a close look at the mechanism for hydrolysis. in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. And typically, water is. What Do Hydrolysis Reactions Do.
From wou.edu
CH103 Chapter 8 The Major Macromolecules Chemistry What Do Hydrolysis Reactions Do hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Hydrolysis is a type of decomposition reaction where one of the reactants is water; in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. A salt is formed between. What Do Hydrolysis Reactions Do.
From www.animalia-life.club
Simple Hydrolysis Reaction What Do Hydrolysis Reactions Do Let’s take a close look at the mechanism for hydrolysis. hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Hydrolysis is a type of decomposition reaction where one of the reactants is water; in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the. What Do Hydrolysis Reactions Do.
From www.slideserve.com
PPT HYDROLYSIS REACTIONS PowerPoint Presentation, free download ID What Do Hydrolysis Reactions Do in its simplest definition, hydrolysis is a chemical reaction in which water is used to break down the bonds of a particular substance. the definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Let’s take a close look at the mechanism for hydrolysis. hydrolysis, in chemistry and physiology, a double decomposition. What Do Hydrolysis Reactions Do.