What Is The Ice Method In Chemistry . I stands for the initial concentrations (or pressures) for each species in the reaction mixture. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. an useful tool in solving equilibrium problems is an ice chart. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. chemical equilibrium (ice method) introduction. the ice table method. 00:00 from initial to equilibrium state; Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and.
from mungfali.com
• chemical equilibrium occurs when opposing reactions are proceeding at equal rates. 00:00 from initial to equilibrium state; ice tables automatically set up and organize the variables and constants needed when calculating the unknown. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. an useful tool in solving equilibrium problems is an ice chart. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. chemical equilibrium (ice method) introduction. the ice table method.
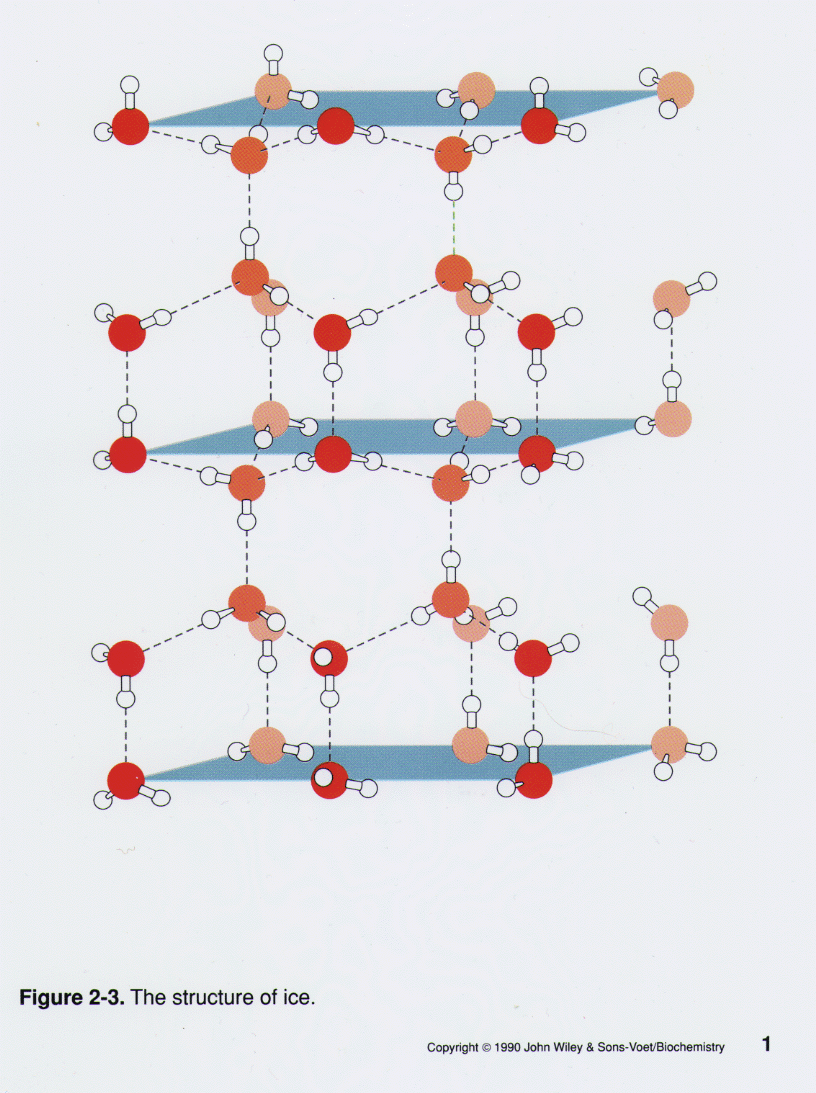
Ice Molecule Structure
What Is The Ice Method In Chemistry an useful tool in solving equilibrium problems is an ice chart. an useful tool in solving equilibrium problems is an ice chart. the ice table method. chemical equilibrium (ice method) introduction. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. 00:00 from initial to equilibrium state; ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and.
From www.youtube.com
Solving Equilibrium ICE Tables WITHOUT the Quadratic Formula YouTube What Is The Ice Method In Chemistry the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. chemical equilibrium (ice method) introduction. 00:00 from initial to equilibrium state; an useful tool in solving equilibrium problems is an ice chart. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. •. What Is The Ice Method In Chemistry.
From www.pinterest.com
Pin on Chemistry Help What Is The Ice Method In Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. 00:00 from initial to equilibrium state; an useful tool in solving equilibrium problems is an ice chart. ice tables automatically set up and organize the variables and constants needed when calculating. What Is The Ice Method In Chemistry.
From www.youtube.com
Equilibrium ICE box YouTube What Is The Ice Method In Chemistry ice tables automatically set up and organize the variables and constants needed when calculating the unknown. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. chemical equilibrium (ice method) introduction. • chemical. What Is The Ice Method In Chemistry.
From www.youtube.com
Chemical equilibrium ICE charts YouTube What Is The Ice Method In Chemistry chemical equilibrium (ice method) introduction. 00:00 from initial to equilibrium state; • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. I stands for. What Is The Ice Method In Chemistry.
From www.youtube.com
AP Chemistry Intro to ICE Charts YouTube What Is The Ice Method In Chemistry ice tables automatically set up and organize the variables and constants needed when calculating the unknown. an useful tool in solving equilibrium problems is an ice chart. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. chemical equilibrium (ice method) introduction. I stands for the initial. What Is The Ice Method In Chemistry.
From www.studocu.com
ICE Method for chemistry CHEM 116 POGIL Discussion Fall 2010 UMass What Is The Ice Method In Chemistry the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. chemical equilibrium (ice method) introduction. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. an useful tool in solving equilibrium. What Is The Ice Method In Chemistry.
From www.youtube.com
Perfect Squares ICE Table Equilibrium Calculations YouTube What Is The Ice Method In Chemistry the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. an useful tool in solving equilibrium problems is an ice chart. chemical equilibrium (ice method) introduction. ice tables automatically set up and. What Is The Ice Method In Chemistry.
From www.showme.com
ICE Table Problem I Science, Chemicalreactions, Chemistry What Is The Ice Method In Chemistry Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. • chemical equilibrium occurs when opposing reactions. What Is The Ice Method In Chemistry.
From www.slideserve.com
PPT Unit 4 Equilibrium PowerPoint Presentation, free download ID What Is The Ice Method In Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. the ice table method. 00:00 from initial to equilibrium state; Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of. What Is The Ice Method In Chemistry.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing What Is The Ice Method In Chemistry chemical equilibrium (ice method) introduction. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. an useful tool in solving equilibrium problems is an ice chart. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice tables (initial, change, equilibrium). What Is The Ice Method In Chemistry.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing What Is The Ice Method In Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. the ice table method. • chemical equilibrium. What Is The Ice Method In Chemistry.
From www.youtube.com
Chemical Equilibrium ICE Table Example 3 YouTube What Is The Ice Method In Chemistry Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. chemical equilibrium (ice method) introduction. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. an useful tool in solving equilibrium problems is. What Is The Ice Method In Chemistry.
From www.sciencephoto.com
Ice, molecular model Stock Image C002/9165 Science Photo Library What Is The Ice Method In Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. chemical equilibrium (ice method) introduction. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. the ice. What Is The Ice Method In Chemistry.
From www.youtube.com
Quadratic Equation ICE Table Equilibrium Calculations YouTube What Is The Ice Method In Chemistry the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. the ice table method. 00:00 from initial to equilibrium state; an useful tool in solving equilibrium problems is an ice chart. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. . What Is The Ice Method In Chemistry.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID3355295 What Is The Ice Method In Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. chemical equilibrium (ice method) introduction. 00:00 from initial to equilibrium state; the ice table method. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice tables (initial, change, equilibrium) are a systematic method to track. What Is The Ice Method In Chemistry.
From www.rechargecolorado.org
How To Use Ice Chart Chemistry Best Picture Of Chart What Is The Ice Method In Chemistry the ice table method. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. an useful tool in solving. What Is The Ice Method In Chemistry.
From www.youtube.com
Chemical Equilibrium Part 2 Equilibrium Calculations (ICE box) YouTube What Is The Ice Method In Chemistry Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. chemical equilibrium (ice method) introduction. the ice table method. ice tables automatically set up and organize the variables and constants needed. What Is The Ice Method In Chemistry.
From chemrxiv.org
HUB A method to model and extract the distribution of ice nucleation What Is The Ice Method In Chemistry the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. chemical equilibrium (ice method) introduction. I. What Is The Ice Method In Chemistry.
From www.pinterest.com
Equilibrium Chemistry Notes, Calculating the Equilibrium Constant (Kc What Is The Ice Method In Chemistry the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. an useful tool in solving equilibrium problems is an ice chart. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. 00:00 from initial to equilibrium state; ice tables automatically set up and organize the variables. What Is The Ice Method In Chemistry.
From www.youtube.com
GCII Calculating equilibrium concentrations using ICE method Part 2 What Is The Ice Method In Chemistry the ice table method. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. an useful tool in solving equilibrium problems is an ice chart. chemical equilibrium (ice method) introduction. . What Is The Ice Method In Chemistry.
From www.youtube.com
Chemical Equilibrium part 3 ICE table YouTube What Is The Ice Method In Chemistry the ice table method. 00:00 from initial to equilibrium state; • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. chemical equilibrium (ice method) introduction. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. ice tables automatically set up and organize the variables and constants needed when calculating. What Is The Ice Method In Chemistry.
From elchoroukhost.net
Ice Table Calculator Online Elcho Table What Is The Ice Method In Chemistry ice tables automatically set up and organize the variables and constants needed when calculating the unknown. 00:00 from initial to equilibrium state; the ice table method. chemical equilibrium (ice method) introduction. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. the acronym ice is commonly used to refer to. What Is The Ice Method In Chemistry.
From www.slideserve.com
PPT The ICE Method …identify… …calm… …exchange… PowerPoint What Is The Ice Method In Chemistry 00:00 from initial to equilibrium state; • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. the ice table method. . What Is The Ice Method In Chemistry.
From www.youtube.com
Chemical Equilibrium ICE Table Example 2 YouTube What Is The Ice Method In Chemistry • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. chemical equilibrium (ice method) introduction. the ice table method. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. an useful tool in. What Is The Ice Method In Chemistry.
From printablelibcohos.z19.web.core.windows.net
Melting Ice In Different Liquids Experiment What Is The Ice Method In Chemistry the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. Ice tables (initial, change, equilibrium) are a systematic method to track. What Is The Ice Method In Chemistry.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube What Is The Ice Method In Chemistry the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. the ice table method. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. chemical equilibrium (ice method) introduction. ice tables automatically set up and organize the variables and constants needed when calculating the unknown.. What Is The Ice Method In Chemistry.
From www.youtube.com
ICE Tables "x is small" Approximation YouTube What Is The Ice Method In Chemistry • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. an useful tool in solving equilibrium problems is an ice chart. 00:00 from initial to equilibrium state; the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. the ice table method. ice tables automatically set. What Is The Ice Method In Chemistry.
From www.showme.com
Ice table intro Science, Chemistry, Equilibrium ShowMe What Is The Ice Method In Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. the ice table method. chemical equilibrium (ice method) introduction. 00:00 from initial to equilibrium state; the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. ice tables automatically set up and organize. What Is The Ice Method In Chemistry.
From www.youtube.com
Application of the "ICE" method in equilibrium calculations YouTube What Is The Ice Method In Chemistry the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. 00:00 from initial to equilibrium state; Ice tables (initial, change, equilibrium). What Is The Ice Method In Chemistry.
From studyhypnotised.z21.web.core.windows.net
When Do You Use Ice Tables What Is The Ice Method In Chemistry • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. the ice table method. chemical equilibrium (ice method) introduction.. What Is The Ice Method In Chemistry.
From sciencenotes.org
Regelation of Ice in Physics and Chemistry What Is The Ice Method In Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. the ice table method. ice tables automatically set up and organize the variables and constants needed when calculating the unknown. chemical equilibrium (ice method) introduction. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. 00:00. What Is The Ice Method In Chemistry.
From www.youtube.com
Equilibrium Reaction with an ICE Table Chemistry Sample Problem YouTube What Is The Ice Method In Chemistry Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. 00:00 from initial to equilibrium state; chemical equilibrium (ice method) introduction. an useful tool in solving equilibrium problems is an ice chart.. What Is The Ice Method In Chemistry.
From elchoroukhost.net
Ice Table Calculator Online Elcho Table What Is The Ice Method In Chemistry the ice table method. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. chemical equilibrium (ice method) introduction. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. 00:00 from initial to equilibrium state; ice tables automatically set up and organize the variables and constants needed when calculating. What Is The Ice Method In Chemistry.
From www.youtube.com
ICE Tables and Quadratic Formula, ICE Tables and Small Equilibrium What Is The Ice Method In Chemistry 00:00 from initial to equilibrium state; Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. ice tables. What Is The Ice Method In Chemistry.
From mungfali.com
Ice Molecule Structure What Is The Ice Method In Chemistry the ice table method. the acronym ice is commonly used to refer to this mathematical approach, and the concentration terms are usually gathered. Ice tables (initial, change, equilibrium) are a systematic method to track the concentrations of reactants and. • chemical equilibrium occurs when opposing reactions are proceeding at equal rates. ice tables automatically set up and. What Is The Ice Method In Chemistry.