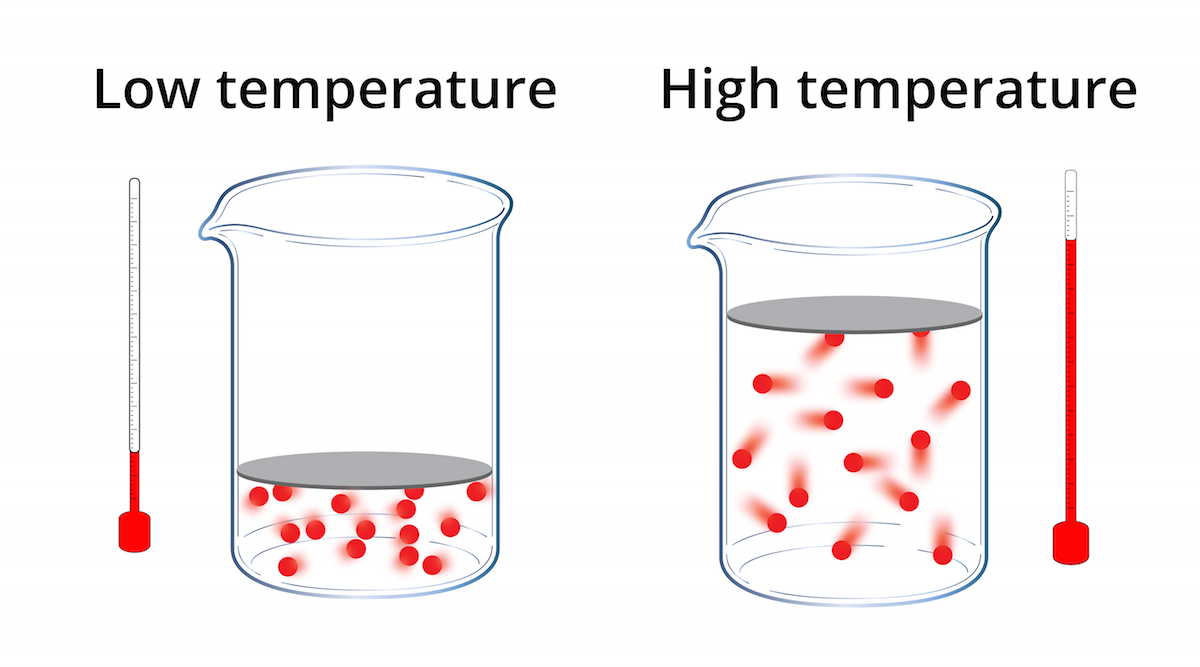
Does High Pressure Change With Temperature . If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take place by reducing volume. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The pressure of the air pushes on the balloon from the inside, causing it to inflate. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. Air pressure depends on the temperature of the air. When you heat the water it expands, which does work against the surrounding. In this scenario, the air is not confined. For water and most solids/liquids, yes but very slightly. If you heat the balloon, the air pressure gets even higher. Weather patterns complicate the relationship between barometric pressure and temperature.
from www.visionlearning.com
If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Weather patterns complicate the relationship between barometric pressure and temperature. The pressure of the air pushes on the balloon from the inside, causing it to inflate. Air pressure depends on the temperature of the air. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. In this scenario, the air is not confined. When you heat the water it expands, which does work against the surrounding. If you heat the balloon, the air pressure gets even higher. In reality, most compression take place by reducing volume. At higher altitudes, the temperature is generally lower, and the air pressure is also lower.
Properties of Gases Chemistry Visionlearning
Does High Pressure Change With Temperature The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. In reality, most compression take place by reducing volume. The pressure of the air pushes on the balloon from the inside, causing it to inflate. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. When you heat the water it expands, which does work against the surrounding. In this scenario, the air is not confined. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. For water and most solids/liquids, yes but very slightly. Weather patterns complicate the relationship between barometric pressure and temperature. Air pressure depends on the temperature of the air. If you heat the balloon, the air pressure gets even higher.
From www.thegeographeronline.net
Weather and Climate THE GEOGRAPHER ONLINE Does High Pressure Change With Temperature When you heat the water it expands, which does work against the surrounding. In this scenario, the air is not confined. Air pressure depends on the temperature of the air. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take place by reducing. Does High Pressure Change With Temperature.
From www.slideserve.com
PPT Section 11.2 Properties of the Atmosphere PowerPoint Does High Pressure Change With Temperature The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. Weather patterns complicate the relationship between barometric pressure and temperature. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. For water and most solids/liquids, yes but. Does High Pressure Change With Temperature.
From mrtirerack.com
Why Does Tire Pressure Change With Temperature The Science Explained Does High Pressure Change With Temperature For water and most solids/liquids, yes but very slightly. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. In this scenario, the air is not confined. The pressure of the air pushes on the balloon from the inside, causing it to inflate. Weather patterns complicate the relationship between barometric pressure and temperature. When you. Does High Pressure Change With Temperature.
From socratic.org
How does temperature affect the atmosphere and cause weather? Socratic Does High Pressure Change With Temperature In reality, most compression take place by reducing volume. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The pressure of the air pushes on the. Does High Pressure Change With Temperature.
From www.kobelco.co.jp
The World of High Temperature and High Pressure KOBE STEEL, LTD. Does High Pressure Change With Temperature For water and most solids/liquids, yes but very slightly. In reality, most compression take place by reducing volume. If you heat the balloon, the air pressure gets even higher. The pressure of the air pushes on the balloon from the inside, causing it to inflate. When you heat the water it expands, which does work against the surrounding. Weather patterns. Does High Pressure Change With Temperature.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does High Pressure Change With Temperature When you heat the water it expands, which does work against the surrounding. Weather patterns complicate the relationship between barometric pressure and temperature. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. In reality, most compression take place by reducing volume. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the. Does High Pressure Change With Temperature.
From www.slideserve.com
PPT Pressure and Temperature PowerPoint Presentation, free download Does High Pressure Change With Temperature The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The pressure of the air pushes on the balloon from the inside, causing it to inflate. In reality, most compression take place by reducing volume. If you heat the balloon, the air. Does High Pressure Change With Temperature.
From www.dreamstime.com
Atmospheric Pressure Example with Lower and Higher Altitude Outline Does High Pressure Change With Temperature In this scenario, the air is not confined. When you heat the water it expands, which does work against the surrounding. Air pressure depends on the temperature of the air. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. Weather patterns complicate the relationship between barometric pressure and temperature. For water and most solids/liquids,. Does High Pressure Change With Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Does High Pressure Change With Temperature In reality, most compression take place by reducing volume. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. For water and most solids/liquids, yes but very slightly. If you heat the balloon, the air pressure gets even higher. Air pressure depends on the temperature of the air. The pressure of the air pushes on. Does High Pressure Change With Temperature.
From www.slideserve.com
PPT Fluid Mechanics PowerPoint Presentation, free download ID436522 Does High Pressure Change With Temperature Air pressure depends on the temperature of the air. When you heat the water it expands, which does work against the surrounding. Weather patterns complicate the relationship between barometric pressure and temperature. In this scenario, the air is not confined. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume,. Does High Pressure Change With Temperature.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Does High Pressure Change With Temperature For water and most solids/liquids, yes but very slightly. Air pressure depends on the temperature of the air. If you heat the balloon, the air pressure gets even higher. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The pressure of the air pushes on the balloon from the inside, causing it to inflate.. Does High Pressure Change With Temperature.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Does High Pressure Change With Temperature At higher altitudes, the temperature is generally lower, and the air pressure is also lower. For water and most solids/liquids, yes but very slightly. Air pressure depends on the temperature of the air. When you heat the water it expands, which does work against the surrounding. If you heat the balloon, the air pressure gets even higher. In reality, most. Does High Pressure Change With Temperature.
From www.slideserve.com
PPT Chapter 6 Air Pressure and Winds PowerPoint Presentation, free Does High Pressure Change With Temperature If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. If you heat the balloon, the air pressure gets even higher. When you heat the water it expands, which does work against the surrounding. The pressure of the air pushes on the balloon from the inside, causing it. Does High Pressure Change With Temperature.
From mrtirerack.com
Why Does Tire Pressure Change With Temperature The Science Explained Does High Pressure Change With Temperature When you heat the water it expands, which does work against the surrounding. Air pressure depends on the temperature of the air. For water and most solids/liquids, yes but very slightly. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If you heat the balloon, the air pressure gets even higher. Weather patterns complicate. Does High Pressure Change With Temperature.
From mungfali.com
High Pressure Weather Diagram Does High Pressure Change With Temperature In this scenario, the air is not confined. In reality, most compression take place by reducing volume. If you heat the balloon, the air pressure gets even higher. Air pressure depends on the temperature of the air. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number. Does High Pressure Change With Temperature.
From courses.lumenlearning.com
Phase Changes Physics Does High Pressure Change With Temperature The pressure of the air pushes on the balloon from the inside, causing it to inflate. Air pressure depends on the temperature of the air. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. For water and most solids/liquids, yes but. Does High Pressure Change With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does High Pressure Change With Temperature The pressure of the air pushes on the balloon from the inside, causing it to inflate. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. If you had a way to increase pressure with no volume change, then yes, temperature would. Does High Pressure Change With Temperature.
From pressbooks.bccampus.ca
LABORATORY 2 HEAT AND TEMPERATURE IN THE ATMOSPHERE Physical Does High Pressure Change With Temperature When you heat the water it expands, which does work against the surrounding. If you heat the balloon, the air pressure gets even higher. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. In reality, most compression take place by reducing. Does High Pressure Change With Temperature.
From www.slideserve.com
PPT Characteristics of the Atmosphere PowerPoint Presentation, free Does High Pressure Change With Temperature At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The pressure of the air pushes on the balloon from the inside, causing it to inflate. When you heat the water it expands,. Does High Pressure Change With Temperature.
From www.slideserve.com
PPT The Atmosphere and Meteorology PowerPoint Presentation, free Does High Pressure Change With Temperature The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. In this scenario, the air is not confined. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The pressure of the air pushes on the balloon. Does High Pressure Change With Temperature.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Does High Pressure Change With Temperature If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If you heat the balloon, the air pressure gets even higher. When you heat the water it expands, which does work against the. Does High Pressure Change With Temperature.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Does High Pressure Change With Temperature In reality, most compression take place by reducing volume. If you heat the balloon, the air pressure gets even higher. For water and most solids/liquids, yes but very slightly. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. Air pressure depends on the temperature of the air. If you had a way to increase. Does High Pressure Change With Temperature.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Does High Pressure Change With Temperature When you heat the water it expands, which does work against the surrounding. Weather patterns complicate the relationship between barometric pressure and temperature. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The pressure of the air pushes on the balloon from the inside, causing it to. Does High Pressure Change With Temperature.
From mungfali.com
High Pressure Weather Diagram Does High Pressure Change With Temperature Air pressure depends on the temperature of the air. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. In this scenario, the air is not confined. When you heat the water it expands, which does work against the surrounding. In reality, most compression take place by reducing volume. The pressure of the air pushes. Does High Pressure Change With Temperature.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does High Pressure Change With Temperature In this scenario, the air is not confined. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Air pressure depends on the temperature of the air. For water and most solids/liquids, yes but very slightly. If you heat the balloon, the air pressure gets even higher. At. Does High Pressure Change With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does High Pressure Change With Temperature The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. Air pressure depends on the temperature of the air. When you heat the water it expands, which does work against the surrounding. In this scenario, the air is not confined. In reality,. Does High Pressure Change With Temperature.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial Does High Pressure Change With Temperature When you heat the water it expands, which does work against the surrounding. Air pressure depends on the temperature of the air. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the. Does High Pressure Change With Temperature.
From www.fiziklah.com
Suhu dan Altitud Fiziklah! Does High Pressure Change With Temperature For water and most solids/liquids, yes but very slightly. Air pressure depends on the temperature of the air. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. Weather patterns complicate the relationship between barometric pressure and temperature. If you had a. Does High Pressure Change With Temperature.
From slideplayer.com
Weather Forecasts. ppt download Does High Pressure Change With Temperature The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. The pressure of the air pushes on the balloon from the inside, causing it to inflate. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. For. Does High Pressure Change With Temperature.
From www.esa.int
ESA Atmospheric temperature changes with altitude Does High Pressure Change With Temperature For water and most solids/liquids, yes but very slightly. If you heat the balloon, the air pressure gets even higher. The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. Weather patterns complicate the relationship between barometric pressure and temperature. At higher. Does High Pressure Change With Temperature.
From www.visionlearning.com
Properties of Gases Chemistry Visionlearning Does High Pressure Change With Temperature Weather patterns complicate the relationship between barometric pressure and temperature. The pressure of the air pushes on the balloon from the inside, causing it to inflate. In this scenario, the air is not confined. If you heat the balloon, the air pressure gets even higher. If you had a way to increase pressure with no volume change, then yes, temperature. Does High Pressure Change With Temperature.
From chem.libretexts.org
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Does High Pressure Change With Temperature In this scenario, the air is not confined. If you heat the balloon, the air pressure gets even higher. The pressure of the air pushes on the balloon from the inside, causing it to inflate. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The thermometer and pressure gauge indicate the temperature and the. Does High Pressure Change With Temperature.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an Does High Pressure Change With Temperature For water and most solids/liquids, yes but very slightly. If you heat the balloon, the air pressure gets even higher. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Weather patterns complicate the relationship between barometric pressure and temperature. In this scenario, the air is not confined.. Does High Pressure Change With Temperature.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Does High Pressure Change With Temperature In reality, most compression take place by reducing volume. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Weather patterns complicate the relationship between barometric pressure and temperature. For water and most. Does High Pressure Change With Temperature.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does High Pressure Change With Temperature The thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask. In reality, most compression take place by reducing volume. Air pressure depends on the temperature of the air. At higher altitudes, the temperature is generally lower, and the air pressure is also. Does High Pressure Change With Temperature.