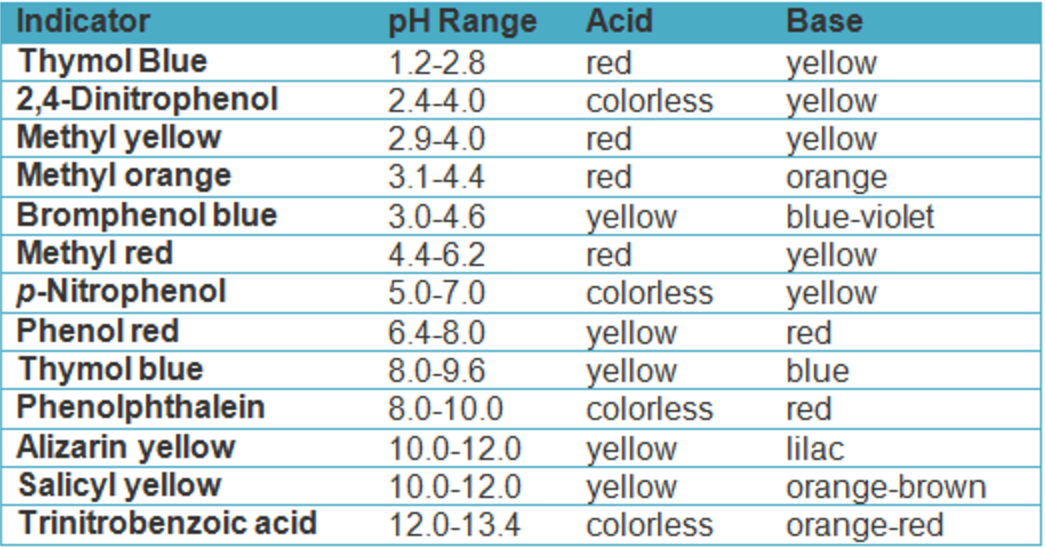
During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction . A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Demonstrate how to select the proper indicator for a titration experiment; Determine the acidic dissociation constants k a or k ai of indicators. They are chosen based on the desired endpoint and the. Using a ph probe and using a colour indicator. The unknown concentration can be calculated using the stoichiometry of the reaction. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. Titrations where a ph probe is.
from classnotes.org.in
Using a ph probe and using a colour indicator. Demonstrate how to select the proper indicator for a titration experiment; A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. Determine the acidic dissociation constants k a or k ai of indicators. There are two basic types of acid base titrations, indicator and potentiometric. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations where a ph probe is. The unknown concentration can be calculated using the stoichiometry of the reaction. They are chosen based on the desired endpoint and the. In an indicator based titration you add another.
Acid Base Titration using Indicator Chemistry, Class 11, Ionic
During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Determine the acidic dissociation constants k a or k ai of indicators. In an indicator based titration you add another. They are chosen based on the desired endpoint and the. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: There are two basic types of acid base titrations, indicator and potentiometric. Demonstrate how to select the proper indicator for a titration experiment; Using a ph probe and using a colour indicator. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. The unknown concentration can be calculated using the stoichiometry of the reaction. Titrations where a ph probe is. Determine the acidic dissociation constants k a or k ai of indicators.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Titrations where a ph probe is. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. In an indicator based titration you add another. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction There are two basic types of acid base titrations, indicator and potentiometric. The unknown concentration can be calculated using the stoichiometry of the reaction. Titrations where a ph probe is. Demonstrate how to select the proper indicator for a titration experiment; Determine the acidic dissociation constants k a or k ai of indicators. They are chosen based on the desired. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.slideserve.com
PPT Neutralization and Titration PowerPoint Presentation, free During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Titrations where a ph probe is. In an indicator based titration you add another. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The unknown concentration can be calculated using the stoichiometry of the reaction. They are chosen based on the desired endpoint and the. There are two basic types. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From mungfali.com
Acid Base Titration Indicator During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Demonstrate how to select the proper indicator for a titration experiment; They are chosen based on the desired endpoint and the. Determine the acidic dissociation constants k a or k ai of indicators. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. The unknown concentration can be calculated using the stoichiometry. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.sliderbase.com
Titration During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. Demonstrate how to select the proper indicator for a titration experiment; For a ph titration (between an acid and a base), there are two common ways to find the. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations where a ph probe is. Demonstrate how to select the proper indicator for a titration experiment; In an indicator based titration you add another. Determine the acidic dissociation constants k a or k ai of indicators. They are chosen based. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Determine the acidic dissociation constants k a or k ai of indicators. The unknown concentration can be calculated using the stoichiometry of the reaction. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: There are two basic types of acid base titrations, indicator and potentiometric. Using a ph probe and. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From slideplayer.com
Video 11.1 Acids and Bases. ppt download During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Determine the acidic dissociation constants k a or k ai of indicators. They are chosen based on the desired endpoint and the. Using a ph probe and using a colour indicator. The unknown concentration can be calculated using the stoichiometry. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From psu.pb.unizin.org
AcidBase Titrations (14.7) Chemistry 110 During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction The unknown concentration can be calculated using the stoichiometry of the reaction. There are two basic types of acid base titrations, indicator and potentiometric. Determine the acidic dissociation constants k a or k ai of indicators. Titrations where a ph probe is. For a ph titration (between an acid and a base), there are two common ways to find the. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.numerade.com
SOLVED EXPERIMENT 3ACID BASE TITRATIONS NEUTRALIZATION REACTION Aim During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Demonstrate how to select the proper indicator for a titration experiment; In an indicator based titration you add another. Using a ph probe and using a colour indicator. There are two basic types of acid base titrations, indicator and potentiometric. Determine the acidic dissociation constants k a or k ai of indicators. A titration’s end point was determined using litmus. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Demonstrate how to select the proper indicator for a titration experiment; Titrations where a ph probe is. Determine the acidic dissociation constants k a or k ai of indicators. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when.. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.tutormyself.com
233 Archives TutorMyself Chemistry During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction They are chosen based on the desired endpoint and the. Using a ph probe and using a colour indicator. In an indicator based titration you add another. The unknown concentration can be calculated using the stoichiometry of the reaction. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration’s. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Using a ph probe and using a colour indicator. Titrations where a ph probe is. Demonstrate how to select the proper indicator for a titration experiment; Determine the acidic dissociation constants k a or k ai of indicators. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The unknown concentration. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Demonstrate how to select the proper indicator for a titration experiment; They are chosen based on the desired endpoint and the. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations where a ph probe is. A titration’s end point was determined using litmus as an indicator, which is red. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.studypool.com
SOLUTION Indicators used in titration Studypool During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. Demonstrate how to select the proper indicator for a titration experiment; They are chosen based on the desired endpoint and the. Titrations where a ph probe is. There are. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction For a ph titration (between an acid and a base), there are two common ways to find the endpoint: In an indicator based titration you add another. Titrations where a ph probe is. They are chosen based on the desired endpoint and the. Demonstrate how to select the proper indicator for a titration experiment; Using a ph probe and using. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.slideserve.com
PPT Neutralization and Titration PowerPoint Presentation, free During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Using a ph probe and using a colour indicator. Demonstrate how to select the proper indicator for a titration experiment; For a ph titration (between an acid and a base), there are two common ways to find the endpoint: In an indicator based titration you add another. Determine the acidic dissociation constants k a or k ai of indicators. A. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction They are chosen based on the desired endpoint and the. Using a ph probe and using a colour indicator. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. There are two basic types of acid base titrations, indicator. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From slideplayer.com
Introduction to Chemical Analysis ppt download During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Using a ph probe and using a colour indicator. Titrations where a ph probe is. The unknown concentration can be calculated using the stoichiometry of the reaction. Determine the acidic dissociation constants k a or k ai of indicators. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: There are. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From dokumen.tips
(PPT) ACID BASE TITRATION INDICATORS. Titration a method of analysis During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction The unknown concentration can be calculated using the stoichiometry of the reaction. Demonstrate how to select the proper indicator for a titration experiment; Titrations where a ph probe is. In an indicator based titration you add another. Using a ph probe and using a colour indicator. A titration’s end point was determined using litmus as an indicator, which is red. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From slidetodoc.com
Unit 11 Acids Bases and Solutions Neutralization Reactions During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction The unknown concentration can be calculated using the stoichiometry of the reaction. They are chosen based on the desired endpoint and the. Using a ph probe and using a colour indicator. Demonstrate how to select the proper indicator for a titration experiment; There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From mavink.com
Titration Reaction During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Titrations where a ph probe is. Using a ph probe and using a colour indicator. Demonstrate how to select the proper indicator for a titration experiment; The unknown concentration can be calculated using the stoichiometry of the reaction. Determine the acidic dissociation constants k a or k ai of indicators. There are two basic types of acid base titrations, indicator. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Titrations where a ph probe is. Using a ph probe and using a colour indicator. In an indicator based titration you add another. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. Demonstrate how to select the proper. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction There are two basic types of acid base titrations, indicator and potentiometric. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The unknown concentration can be calculated using the stoichiometry of the reaction. Demonstrate how to select the proper indicator for a titration experiment; In an indicator based titration you. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction The unknown concentration can be calculated using the stoichiometry of the reaction. Demonstrate how to select the proper indicator for a titration experiment; Using a ph probe and using a colour indicator. They are chosen based on the desired endpoint and the. Titrations where a ph probe is. In an indicator based titration you add another. Determine the acidic dissociation. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.microlit.com
An Advanced Guide to Titration Microlit During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Titrations where a ph probe is. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. Determine the acidic dissociation constants k a or k ai of indicators. They are chosen based on the desired endpoint and the. Demonstrate. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Using a ph probe and using a colour indicator. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. Determine the acidic dissociation constants k a or k ai of indicators. Demonstrate how to select the proper indicator for. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From slideplayer.com
Titrations!. ppt download During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations where a ph probe is. Determine the acidic dissociation constants k a or k ai of indicators. There are two basic types of acid base titrations, indicator and potentiometric. The unknown. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Determine the acidic dissociation constants k a or k ai of indicators. The unknown concentration can be calculated using the stoichiometry of the reaction. Titrations where a ph probe is. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: They are chosen based on the desired endpoint and the. Demonstrate. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction They are chosen based on the desired endpoint and the. In an indicator based titration you add another. Using a ph probe and using a colour indicator. Titrations where a ph probe is. There are two basic types of acid base titrations, indicator and potentiometric. A titration’s end point was determined using litmus as an indicator, which is red in. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction The unknown concentration can be calculated using the stoichiometry of the reaction. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. In an indicator based titration you add another. Demonstrate how to select the proper indicator for a. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From slideplayer.com
Concentration of Solutions ppt download During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Determine the acidic dissociation constants k a or k ai of indicators. In an indicator based titration you add another. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when. There are two basic types of acid base titrations,. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From slideplayer.com
Chapter 14 Acids and Bases ppt download During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction The unknown concentration can be calculated using the stoichiometry of the reaction. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: They are chosen based on the desired endpoint and the. Demonstrate how to select the proper indicator for a titration experiment; Using a ph probe and using a colour. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction Determine the acidic dissociation constants k a or k ai of indicators. There are two basic types of acid base titrations, indicator and potentiometric. Titrations where a ph probe is. In an indicator based titration you add another. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions,. During A Titration Indicators Are Used To Determine The Endpoint Of A Neutralization Reaction.