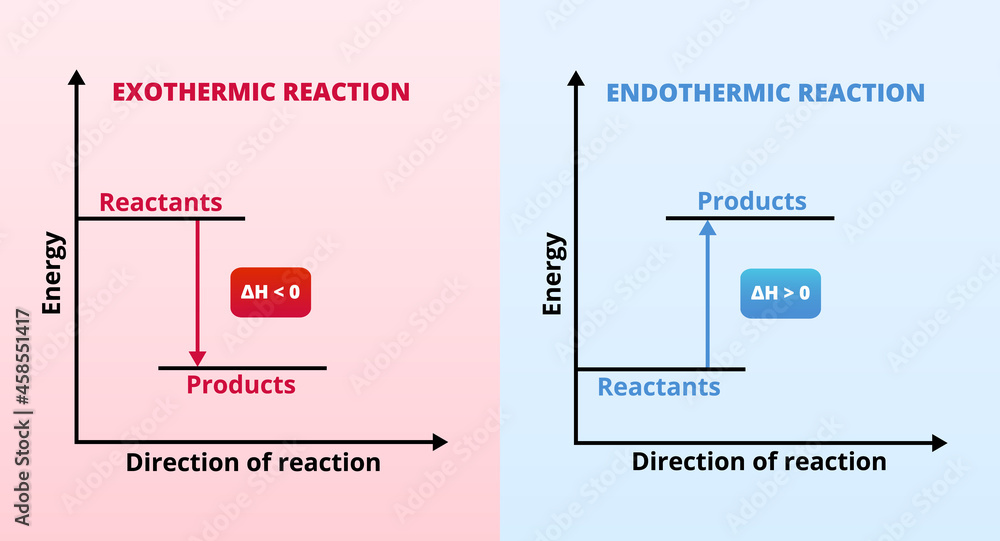
Endothermic Reaction Vs Exothermic . Gibbs free energy and spontaneity. Read on to learn about how to. When a chemical reaction happens, energy is transferred to or from the surroundings. This can be seen by a drop in the medium’s temperature. When energy is transferred to the surroundings, this is called an exothermic reaction and. A look at a seductive. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Photosynthesis is a good example of an endothermic. More rigorous gibbs free energy / spontaneity relationship. Exothermic and endothermic reactions cause. Reactions can either generate or consume energy in the form of heat.
from
Photosynthesis is a good example of an endothermic. A look at a seductive. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Reactions can either generate or consume energy in the form of heat. Exothermic and endothermic reactions cause. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. When a chemical reaction happens, energy is transferred to or from the surroundings. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Gibbs free energy and spontaneity. More rigorous gibbs free energy / spontaneity relationship.
Endothermic Reaction Vs Exothermic When a chemical reaction happens, energy is transferred to or from the surroundings. A look at a seductive. Reactions can either generate or consume energy in the form of heat. When a chemical reaction happens, energy is transferred to or from the surroundings. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Exothermic and endothermic reactions cause. More rigorous gibbs free energy / spontaneity relationship. When energy is transferred to the surroundings, this is called an exothermic reaction and. This can be seen by a drop in the medium’s temperature. Gibbs free energy and spontaneity. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Read on to learn about how to. Photosynthesis is a good example of an endothermic.
From www.freepik.com
Premium Vector Enthalpy endothermic versus exothermic reactions Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. More rigorous gibbs free energy / spontaneity relationship. Read on to learn about how to. A look at a seductive. Reactions can either generate or consume energy in the form of heat. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Endothermic and. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Reactions can either generate or consume energy in the form of heat. A look at a seductive. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. This can be seen by a drop in the medium’s temperature. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Exothermic and endothermic reactions cause. When. Endothermic Reaction Vs Exothermic.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Vs Exothermic Reactions can either generate or consume energy in the form of heat. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. When a chemical reaction happens, energy is transferred to or from the surroundings. More rigorous gibbs free energy / spontaneity relationship. Photosynthesis is a good example of an endothermic. A look at a. Endothermic Reaction Vs Exothermic.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Vs Exothermic When a chemical reaction happens, energy is transferred to or from the surroundings. This can be seen by a drop in the medium’s temperature. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Reactions can either generate or consume energy in the form of heat. Endothermic reactions are reactions that lower the energy level of the. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Exothermic and endothermic reactions cause. When energy is transferred to the surroundings, this is called an exothermic reaction and. Reactions can either generate or consume energy in the form of heat. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Read on to learn about how to. This can be seen by a drop. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Exothermic and endothermic reactions cause. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). When energy is transferred to the surroundings, this is called an exothermic reaction and.. Endothermic Reaction Vs Exothermic.
From www.pinterest.com
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Endothermic Reaction Vs Exothermic Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Gibbs free energy and spontaneity. When energy is transferred to the surroundings, this is called an exothermic reaction and. Exothermic and endothermic reactions cause. This can be seen by a drop in the medium’s temperature. A look at a seductive. Endothermic reactions are reactions that lower the. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. When a chemical reaction happens, energy is transferred to or from the surroundings. When energy is transferred to the surroundings, this is called an exothermic reaction and. Read on to learn about how to. Gibbs free energy and spontaneity. This can be seen by a drop in. Endothermic Reaction Vs Exothermic.
From quizzcampusduran101.z13.web.core.windows.net
Endothermic Versus Exothermic Reaction Endothermic Reaction Vs Exothermic Reactions can either generate or consume energy in the form of heat. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. This can be seen by a drop in the medium’s temperature. Endothermic reactions result in an overall positive heat of. Endothermic Reaction Vs Exothermic.
From lessonpage.z13.web.core.windows.net
Endothermic Vs Exothermic Examples Chemistry Endothermic Reaction Vs Exothermic Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Photosynthesis is a good example of an endothermic. Gibbs free energy and spontaneity. When a chemical reaction happens, energy is transferred to or from the surroundings. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. When energy is transferred to the surroundings, this. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. More rigorous gibbs free energy / spontaneity relationship. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Read on to learn about how to. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Gibbs free energy and spontaneity. Exothermic and endothermic. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic More rigorous gibbs free energy / spontaneity relationship. This can be seen by a drop in the medium’s temperature. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Exothermic and endothermic reactions cause. Read on to learn about how to. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). When. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. Photosynthesis is a good example of an endothermic. When a chemical reaction happens, energy is transferred to or from the surroundings. Read on to learn about how to. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Gibbs free energy and spontaneity. When. Endothermic Reaction Vs Exothermic.
From mungfali.com
Endothermic Vs Exothermic Reactions Examples Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. A look at a seductive. When energy is transferred to the surroundings, this is called an exothermic reaction and. Gibbs free energy and spontaneity. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Read on to learn about how to. When a chemical reaction happens,. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic When a chemical reaction happens, energy is transferred to or from the surroundings. More rigorous gibbs free energy / spontaneity relationship. Gibbs free energy and spontaneity. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. This can be seen by a drop in the medium’s temperature. Read on. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Gibbs free energy and spontaneity. When a chemical reaction happens, energy is transferred to or from the surroundings. Reactions can either generate or consume energy in the form of heat. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Exothermic and endothermic reactions cause. Read on to learn about how to. Endothermic reactions result in an. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic More rigorous gibbs free energy / spontaneity relationship. A look at a seductive. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Reactions can either generate or consume energy in the form of heat. Read on to learn about how to. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy.. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic When a chemical reaction happens, energy is transferred to or from the surroundings. Gibbs free energy and spontaneity. When energy is transferred to the surroundings, this is called an exothermic reaction and. A look at a seductive. Exothermic and endothermic reactions cause. Photosynthesis is a good example of an endothermic. Read on to learn about how to. Endothermic reactions are. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Gibbs free energy and spontaneity. When energy is transferred to the surroundings, this is called an exothermic reaction and. More rigorous gibbs free energy / spontaneity relationship. Endothermic reactions result in an overall positive heat of. Endothermic Reaction Vs Exothermic.
From www.youtube.com
Endothermic vs Exothermic Reactions YouTube Endothermic Reaction Vs Exothermic When energy is transferred to the surroundings, this is called an exothermic reaction and. Photosynthesis is a good example of an endothermic. Exothermic and endothermic reactions cause. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Read on to learn about how to. A look at a seductive. Endothermic reactions are reactions that lower the energy. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. More rigorous gibbs free energy / spontaneity relationship. Reactions can either generate or consume energy in the form of heat. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Exothermic and endothermic reactions cause. Photosynthesis is a good example of an endothermic. Endothermic and exothermic. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Read on to learn about how to. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Reactions can either generate or consume energy in the form of heat.. Endothermic Reaction Vs Exothermic.
From www.youtube.com
Exothermic vs Endothermic Chemical Reactions YouTube Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. Gibbs free energy and spontaneity. When energy is transferred to the surroundings, this is called an exothermic reaction and. Reactions can either generate or consume energy in the form of heat. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Exothermic and endothermic reactions cause.. Endothermic Reaction Vs Exothermic.
From stock.adobe.com
Vecteur Stock Vector graphs or charts of endothermic and exothermic Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Reactions can either generate or consume energy in the form of heat. Read on to learn about how to. When energy is transferred to the surroundings, this is called an exothermic reaction and. A. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Reactions can either generate or consume energy in the form of heat. When a chemical reaction happens, energy is transferred to or from the surroundings. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. More rigorous gibbs free energy / spontaneity relationship. When energy is transferred to the. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic More rigorous gibbs free energy / spontaneity relationship. When a chemical reaction happens, energy is transferred to or from the surroundings. Photosynthesis is a good example of an endothermic. This can be seen by a drop in the medium’s temperature. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. A look at a seductive.. Endothermic Reaction Vs Exothermic.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction Vs Exothermic When a chemical reaction happens, energy is transferred to or from the surroundings. Reactions can either generate or consume energy in the form of heat. A look at a seductive. Gibbs free energy and spontaneity. Exothermic and endothermic reactions cause. More rigorous gibbs free energy / spontaneity relationship. Read on to learn about how to. Endothermic reactions result in an. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic When energy is transferred to the surroundings, this is called an exothermic reaction and. Gibbs free energy and spontaneity. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Read on to learn about how to. Reactions can either generate or consume. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Gibbs free energy and spontaneity. A look at a seductive. This can be seen by a drop in the medium’s temperature. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Read on to learn about how to. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Photosynthesis is a good. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. Gibbs free energy and spontaneity. When a chemical reaction happens, energy is transferred to or from the surroundings. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. When energy is transferred to the surroundings, this is called an exothermic reaction and. More rigorous gibbs free. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Exothermic and endothermic reactions cause. Read on to learn about how to. When energy is transferred to the surroundings, this is called an exothermic reaction and. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Gibbs free energy and spontaneity. Photosynthesis is a. Endothermic Reaction Vs Exothermic.
From www.pinterest.ph
Endothermic and Exothermic Reactions infographic diagram showing Endothermic Reaction Vs Exothermic Gibbs free energy and spontaneity. Photosynthesis is a good example of an endothermic. Exothermic and endothermic reactions cause. Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. A look at a seductive. When a chemical reaction happens, energy is transferred to or from the surroundings. This can be seen by a drop in the. Endothermic Reaction Vs Exothermic.
From
Endothermic Reaction Vs Exothermic Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. This can be seen by a drop in the medium’s temperature. Photosynthesis is a good example of an endothermic. Exothermic and endothermic reactions cause. More rigorous gibbs free energy / spontaneity relationship. Gibbs free energy and spontaneity. Read on to learn about how to. Endothermic. Endothermic Reaction Vs Exothermic.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube Endothermic Reaction Vs Exothermic Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. Exothermic and endothermic reactions cause. Photosynthesis is a good example of an endothermic. More rigorous gibbs free energy / spontaneity relationship. When a chemical reaction happens, energy is transferred to or from the surroundings. When energy is transferred to the surroundings, this is called an. Endothermic Reaction Vs Exothermic.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Endothermic Reaction Vs Exothermic This can be seen by a drop in the medium’s temperature. Exothermic and endothermic reactions cause. A look at a seductive. Read on to learn about how to. Endothermic reactions result in an overall positive heat of reaction (\(q_{rxn} > 0\)). Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. More rigorous gibbs free energy /. Endothermic Reaction Vs Exothermic.