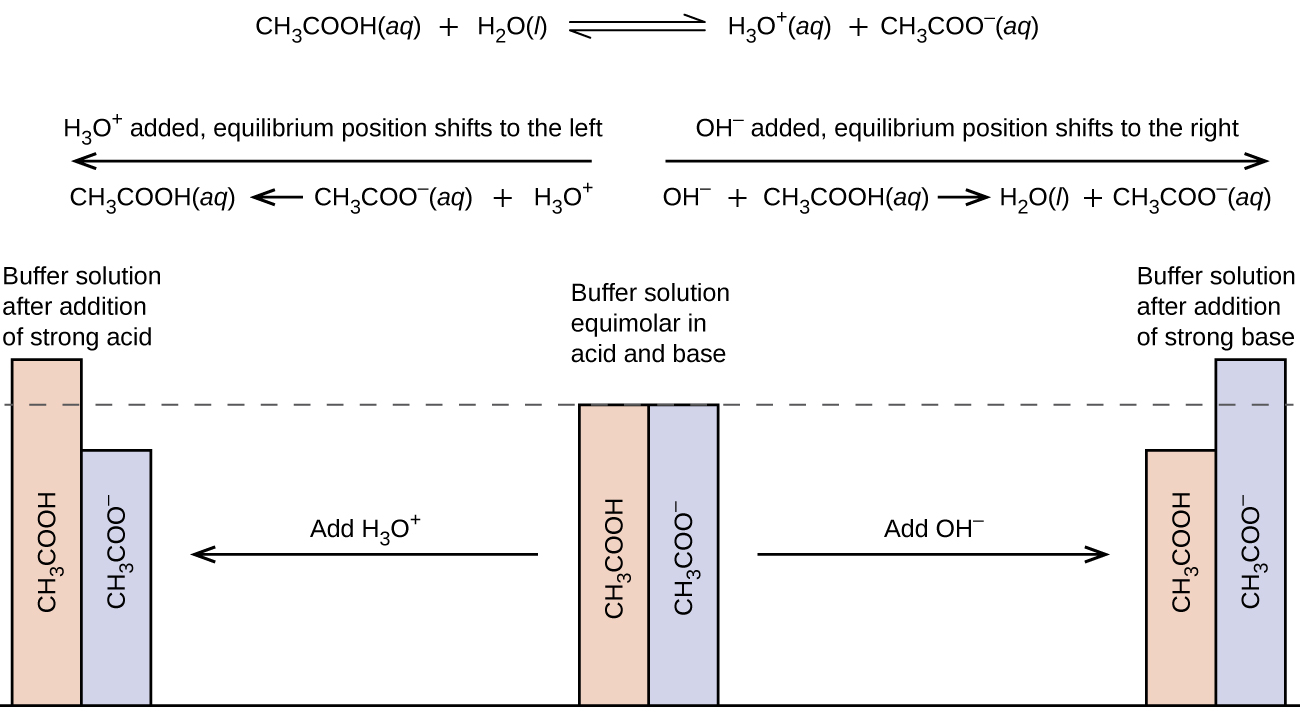
What Is A Buffer System . Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. Learn how buffers work, what types of buffers exist, and why they are. It is able to neutralize small. A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. Learn about the characteristics, types, actions, and. A buffer is a solution that resists ph change when small amounts of acid or base are added. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Learn how to form it, along with its uses & applications. Buffer solution meaning & explanation with examples & chemical equations. How to calculate & find its ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn how buffers work, how to select and calculate them, and what are some common biological and chemical buffers. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid.
from
A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. Learn about the characteristics, types, actions, and. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Learn how to form it, along with its uses & applications. How to calculate & find its ph. Learn how buffers work, what types of buffers exist, and why they are. A buffer is a solution that resists ph change when small amounts of acid or base are added. A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases.
What Is A Buffer System Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. Learn how buffers work, how to select and calculate them, and what are some common biological and chemical buffers. Buffer solution meaning & explanation with examples & chemical equations. Learn how buffers work, what types of buffers exist, and why they are. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Learn how to form it, along with its uses & applications. Learn about the characteristics, types, actions, and. A buffer is a solution that resists ph change when small amounts of acid or base are added. How to calculate & find its ph.
From
What Is A Buffer System A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Learn how to form it, along with its uses & applications. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph. What Is A Buffer System.
From
What Is A Buffer System Learn how buffers work, what types of buffers exist, and why they are. Learn how to form it, along with its uses & applications. A buffer is a solution that resists ph change when small amounts of acid or base are added. A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. Learn how buffers work,. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that resists ph change when small amounts of acid or base are added. It is able to neutralize small. Buffer solution meaning & explanation with examples & chemical equations. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by. What Is A Buffer System.
From
What Is A Buffer System How to calculate & find its ph. Learn how buffers work, how to select and calculate them, and what are some common biological and chemical buffers. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn about the characteristics, types, actions, and. Learn how buffers work, what types of buffers. What Is A Buffer System.
From
What Is A Buffer System Learn how to form it, along with its uses & applications. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Learn about the characteristics, types, actions, and. Buffer solution meaning & explanation with examples & chemical equations. A buffer is a solution that maintains a constant hydrogen. What Is A Buffer System.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is A Buffer System How to calculate & find its ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn how buffers work, how to select and calculate them, and what are some common biological and chemical buffers. Buffer solution meaning & explanation with examples & chemical equations. It is able to neutralize. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn how buffers work, what types of buffers exist, and why they are. Learn how to form it, along with its uses & applications. Learn about the characteristics, types, actions, and. Learn how buffers work, how to select and calculate them,. What Is A Buffer System.
From animalia-life.club
Phosphate Buffer System Equation What Is A Buffer System Learn how buffers work, what types of buffers exist, and why they are. Learn about the characteristics, types, actions, and. A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. It is able to neutralize small. A buffer is a mixture of a weak acid and its conjugate base (or a. What Is A Buffer System.
From
What Is A Buffer System A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. Learn how buffers work, what types of buffers exist, and why they are. A buffer is a solution that resists ph change when small amounts of. What Is A Buffer System.
From study.com
Buffer System in Chemistry Definition & Overview Video & Lesson What Is A Buffer System Learn about the characteristics, types, actions, and. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. A buffer is a solution that resists ph change when small amounts of acid or base are added. Learn. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. Learn how to form it, along with. What Is A Buffer System.
From animalia-life.club
Phosphate Buffer System Equation What Is A Buffer System It is able to neutralize small. Buffer solution meaning & explanation with examples & chemical equations. A buffer is a solution that resists ph change when small amounts of acid or base are added. Learn about the characteristics, types, actions, and. Learn how buffers work, how to select and calculate them, and what are some common biological and chemical buffers.. What Is A Buffer System.
From
What Is A Buffer System Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. How to calculate & find its ph. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or. What Is A Buffer System.
From
What Is A Buffer System Learn how buffers work, how to select and calculate them, and what are some common biological and chemical buffers. Learn about the characteristics, types, actions, and. How to calculate & find its ph. A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. Buffers are characterized by the ph range over. What Is A Buffer System.
From
What Is A Buffer System Learn how to form it, along with its uses & applications. How to calculate & find its ph. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. A buffer is a solution that maintains a constant hydrogen. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its. What Is A Buffer System.
From
What Is A Buffer System How to calculate & find its ph. Learn how buffers work, how to select and calculate them, and what are some common biological and chemical buffers. Learn how buffers work, what types of buffers exist, and why they are. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid). What Is A Buffer System.
From
What Is A Buffer System How to calculate & find its ph. Learn how to form it, along with its uses & applications. It is able to neutralize small. Buffer solution meaning & explanation with examples & chemical equations. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in. What Is A Buffer System.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching What Is A Buffer System Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. A buffer is a solution that resists ph change. What Is A Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Is A Buffer System Learn how to form it, along with its uses & applications. Learn how buffers work, how to select and calculate them, and what are some common biological and chemical buffers. It is able to neutralize small. How to calculate & find its ph. Learn about the characteristics, types, actions, and. A buffer is a solution that maintains a constant hydrogen. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. Learn how to form it, along with its uses & applications. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a mixture of a weak. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. Learn about the characteristics, types, actions, and. How to calculate & find its ph. Learn how to form it, along with its uses & applications. Learn how buffers work, how to select and calculate them, and what are some common biological. What Is A Buffer System.
From www.slideserve.com
PPT Acid and Base Balance PowerPoint Presentation, free download ID What Is A Buffer System Buffer solution meaning & explanation with examples & chemical equations. It is able to neutralize small. Learn how buffers work, what types of buffers exist, and why they are. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains a constant hydrogen ion concentration. What Is A Buffer System.
From
What Is A Buffer System It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that resists ph change when small amounts of acid or base are added. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Learn. What Is A Buffer System.
From
What Is A Buffer System Learn about the characteristics, types, actions, and. Learn how buffers work, what types of buffers exist, and why they are. Learn how to form it, along with its uses & applications. Buffer solution meaning & explanation with examples & chemical equations. How to calculate & find its ph. It is able to neutralize small. A buffer is a mixture of. What Is A Buffer System.
From
What Is A Buffer System It is able to neutralize small. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. How to calculate & find its ph. A buffer is a solution that can resist ph change upon the addition. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn how buffers work, how to select and calculate them, and what are some common biological and chemical buffers. Learn how to form it, along with its uses & applications. A buffer is a solution that maintains a constant hydrogen ion. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. How to calculate & find its ph. Learn about the characteristics, types, actions, and. A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or. What Is A Buffer System.
From
What Is A Buffer System How to calculate & find its ph. A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. Buffer solution meaning & explanation with examples & chemical equations. A buffer is a solution that resists ph change. What Is A Buffer System.
From www.slideserve.com
PPT Buffer Systems PowerPoint Presentation, free download ID9400078 What Is A Buffer System A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. Learn how to form it, along with its uses & applications. Learn about the characteristics, types, actions, and. A buffer is a solution that maintains the. What Is A Buffer System.
From
What Is A Buffer System A buffer is a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) that resists a change in ph when small amounts of a strong acid. A buffer is a solution that resists ph change when small amounts of acid or base are added. A buffer is a solution that maintains the. What Is A Buffer System.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and What Is A Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can. A buffer is. What Is A Buffer System.
From
What Is A Buffer System A buffer is a solution that maintains a constant hydrogen ion concentration in the presence of acids or bases. It is able to neutralize small. Buffer solution meaning & explanation with examples & chemical equations. Learn how buffers work, what types of buffers exist, and why they are. Buffers are characterized by the ph range over which they can maintain. What Is A Buffer System.