Standard Electrode Potential Byju's . The potential difference that is generated is known as the electrode potential. It is also called standard reduction potential. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Electrode potential is a measure of reducing power of any element. The iupac gold book defines it. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: If we take copper and place it in a solution in the. Standard hydrogen electrode is used as a reference. We will also look at some examples to understand the concept a little better.
from askfilo.com
Standard hydrogen electrode is used as a reference. We will also look at some examples to understand the concept a little better. The iupac gold book defines it. The potential difference that is generated is known as the electrode potential. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: If we take copper and place it in a solution in the. Electrode potential is a measure of reducing power of any element. It is also called standard reduction potential.
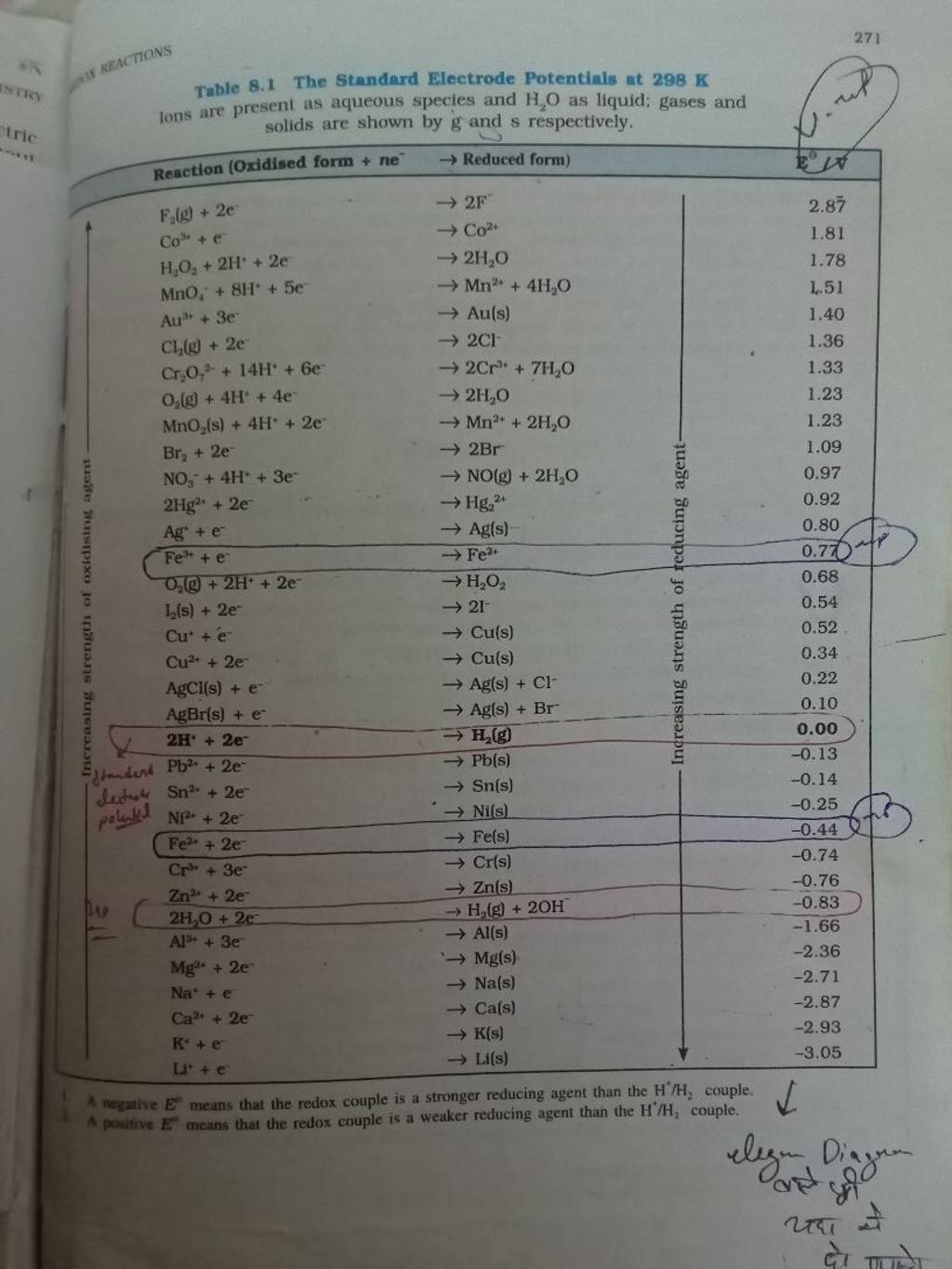
271 Table 8.1 The Standard Electrode Potentials at 298 K lons are present..
Standard Electrode Potential Byju's If we take copper and place it in a solution in the. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: Standard hydrogen electrode is used as a reference. It is also called standard reduction potential. The potential difference that is generated is known as the electrode potential. Electrode potential is a measure of reducing power of any element. The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. If we take copper and place it in a solution in the. We will also look at some examples to understand the concept a little better.
From gamma.app
Understanding Standard Electrode Potential and Mean Activity Coefficient Standard Electrode Potential Byju's 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: We will also look at some examples to understand the concept a little better. Electrode potential is a measure of reducing power of any element. If we take copper and place it in a solution in the. It is. Standard Electrode Potential Byju's.
From byjus.com
Detail explanation about positive and negative value of electrode Standard Electrode Potential Byju's In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Electrode potential is a measure of reducing power of any element. Standard hydrogen electrode is used as a reference. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Standard Electrode Potential Byju's.
From byjus.com
Electrode potential,standard potential and reduction potential are Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. We will also look at some examples to understand the concept a little better. If we take copper and place it in a solution in the. The iupac gold book defines it. It is also called standard reduction potential. In electrochemistry, standard electrode potential , or , is a measure of the. Standard Electrode Potential Byju's.
From scienceinfo.com
Measuring the Standard Electrode Potential (Eꝋ) Standard Electrode Potential Byju's 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: The potential difference that is generated is known as the electrode potential. It is also called standard reduction potential. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound.. Standard Electrode Potential Byju's.
From byjus.com
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe Standard Electrode Potential Byju's If we take copper and place it in a solution in the. The potential difference that is generated is known as the electrode potential. We will also look at some examples to understand the concept a little better. Standard hydrogen electrode is used as a reference. It is also called standard reduction potential. 372 rows the data below tabulates standard. Standard Electrode Potential Byju's.
From cemubadx.blob.core.windows.net
Standard Cell Potential And Standard Electrode Potential at Nina Standard Electrode Potential Byju's 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: Standard hydrogen electrode is used as a reference. We will also look at some examples to understand the concept a little better. The iupac gold book defines it. If we take copper and place it in a solution in. Standard Electrode Potential Byju's.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. We will also look at some examples to understand the concept a little better. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The potential difference that is generated is known as the electrode potential. The iupac gold book defines it.. Standard Electrode Potential Byju's.
From askfilo.com
271 Table 8.1 The Standard Electrode Potentials at 298 K lons are present.. Standard Electrode Potential Byju's In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. If we take copper and place it in a solution in the. Standard hydrogen electrode is used as a reference. It is also called standard reduction potential. The potential difference that is generated is known as the electrode potential. The. Standard Electrode Potential Byju's.
From www.scribd.com
Factor Affecting Standard Electrode Potential PDF Standard Electrode Potential Byju's It is also called standard reduction potential. If we take copper and place it in a solution in the. The potential difference that is generated is known as the electrode potential. The iupac gold book defines it. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: We will. Standard Electrode Potential Byju's.
From users.highland.edu
Standard Potentials Standard Electrode Potential Byju's It is also called standard reduction potential. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The potential difference that is generated is known as the electrode potential. If we take copper and place it in a solution in the. Electrode potential is a measure of reducing power of. Standard Electrode Potential Byju's.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Byju's It is also called standard reduction potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: We will also look at some examples to understand the concept a little better. Electrode potential is a measure of reducing power of any element. The iupac gold book defines it. Standard. Standard Electrode Potential Byju's.
From www.chegg.com
Solved 7.17 From the standard electrode potentials in Table Standard Electrode Potential Byju's The potential difference that is generated is known as the electrode potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: The iupac gold book defines it. If we take copper and place it in a solution in the. In electrochemistry, standard electrode potential , or , is. Standard Electrode Potential Byju's.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5368514 Standard Electrode Potential Byju's It is also called standard reduction potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: The iupac gold book defines it. Electrode potential is a measure of reducing power of any element. In electrochemistry, standard electrode potential , or , is a measure of the reducing power. Standard Electrode Potential Byju's.
From pandai.me
Standard Electrode Potential Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. The iupac gold book defines it. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: If we take copper and place it in a solution in the. Electrode potential is a measure of reducing power of any element. In electrochemistry,. Standard Electrode Potential Byju's.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Standard Electrode Potential Byju's If we take copper and place it in a solution in the. We will also look at some examples to understand the concept a little better. Electrode potential is a measure of reducing power of any element. The potential difference that is generated is known as the electrode potential. The iupac gold book defines it. In electrochemistry, standard electrode potential. Standard Electrode Potential Byju's.
From www.youtube.com
Electrode Potential Standard Electrode potential Reduction potential Standard Electrode Potential Byju's Electrode potential is a measure of reducing power of any element. It is also called standard reduction potential. The iupac gold book defines it. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: In electrochemistry, standard electrode potential , or , is a measure of the reducing power. Standard Electrode Potential Byju's.
From cemubadx.blob.core.windows.net
Standard Cell Potential And Standard Electrode Potential at Nina Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. Electrode potential is a measure of reducing power of any element. The potential difference that is generated is known as the electrode potential. It is also called standard reduction potential. The iupac gold book defines it. We will also look at some examples to understand the concept a little better. If we. Standard Electrode Potential Byju's.
From app.pandai.org
Standard Electrode Potential Standard Electrode Potential Byju's In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: It is also called standard reduction potential. If we take copper and place it in a solution in the.. Standard Electrode Potential Byju's.
From www.studocu.com
Standard Electrode Potentials Electrochemistry Studocu Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. The potential difference that is generated is known as the electrode potential. It is also called standard reduction potential. If we take copper and place it in a solution in the. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Electrode. Standard Electrode Potential Byju's.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Electrode Potential Byju's The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. We will also look at some examples to understand the concept a little better. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she),. Standard Electrode Potential Byju's.
From www.pveducation.org
Standard Potential PVEducation Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. Electrode potential is a measure of reducing power of any element. The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to. Standard Electrode Potential Byju's.
From mungfali.com
Electrode Potential Series Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. The potential difference that is generated is known as the electrode potential. We will also look at some examples to understand the concept a little better. The iupac gold book defines it. Electrode potential is a measure of reducing power of any element. It is also called standard reduction potential. 372 rows. Standard Electrode Potential Byju's.
From www.youtube.com
Types of Electrode Potential Class 11 & 12 CHEMISTRY JEE 2021 JEE Standard Electrode Potential Byju's In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The iupac gold book defines it. We will also look at some examples to understand the concept a little better. It is also called standard reduction potential. The potential difference that is generated is known as the electrode potential. 372. Standard Electrode Potential Byju's.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential Byju's In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. We will also look at some examples to understand the concept a little better. The iupac gold book defines it. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she),. Standard Electrode Potential Byju's.
From mungfali.com
Standard Electrode Potential Table Standard Electrode Potential Byju's The iupac gold book defines it. We will also look at some examples to understand the concept a little better. It is also called standard reduction potential. Electrode potential is a measure of reducing power of any element. The potential difference that is generated is known as the electrode potential. If we take copper and place it in a solution. Standard Electrode Potential Byju's.
From byjus.com
Standard electrode potentials are Fe2+/Fe; E° = 0.44 volts Fe3+/Fe2+; E Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: It is also called standard reduction potential. The iupac gold book defines it. Electrode potential is a measure of reducing power of any element. If we take copper and place it. Standard Electrode Potential Byju's.
From studylib.net
19 Applications of Standard Electrode Potentials Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. The potential difference that is generated is known as the electrode potential. We will also look at some examples to understand the concept a little better. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: The iupac gold book defines. Standard Electrode Potential Byju's.
From testbook.com
Standard Electrode PotentialLearn Definition,Formula,Conditions Standard Electrode Potential Byju's Electrode potential is a measure of reducing power of any element. Standard hydrogen electrode is used as a reference. The iupac gold book defines it. We will also look at some examples to understand the concept a little better. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Standard Electrode Potential Byju's.
From dxozzzxem.blob.core.windows.net
Standard Electrode Potential Are Fe2+/Fe at Frances Howell blog Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. It is also called standard reduction potential. We will also look at some examples to understand the concept a little better. The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The potential difference that is. Standard Electrode Potential Byju's.
From saylordotorg.github.io
Standard Potentials Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. If we take copper and place it in a solution in the. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or. Standard Electrode Potential Byju's.
From byjus.com
Standard electrode potentials are Fe2+/Fe; E° = 0.44 volts Fe3+/Fe2+; E Standard Electrode Potential Byju's 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: Standard hydrogen electrode is used as a reference. If we take copper and place it in a solution in the. The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of the. Standard Electrode Potential Byju's.
From www.pdffiller.com
Electrode Standard Electrode Potential Byju's Standard hydrogen electrode is used as a reference. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: If we take copper and place it in a solution in the. The iupac gold book defines it. The potential difference that is generated is known as the electrode potential. In. Standard Electrode Potential Byju's.
From www.youtube.com
Standard electrode potential \( \left(E^{\circ}\right) \) for Standard Electrode Potential Byju's 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: If we take copper and place it in a solution in the. The iupac gold book defines it. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. It. Standard Electrode Potential Byju's.
From www.youtube.com
APSC132 lecture 5 03 Standard Electrode Potential YouTube Standard Electrode Potential Byju's We will also look at some examples to understand the concept a little better. The potential difference that is generated is known as the electrode potential. It is also called standard reduction potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: Standard hydrogen electrode is used as. Standard Electrode Potential Byju's.
From www.toppr.com
Using the standard electrode potentials given in the table, predict the Standard Electrode Potential Byju's 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at: Standard hydrogen electrode is used as a reference. If we take copper and place it in a solution in the. The potential difference that is generated is known as the electrode potential. Electrode potential is a measure of reducing. Standard Electrode Potential Byju's.