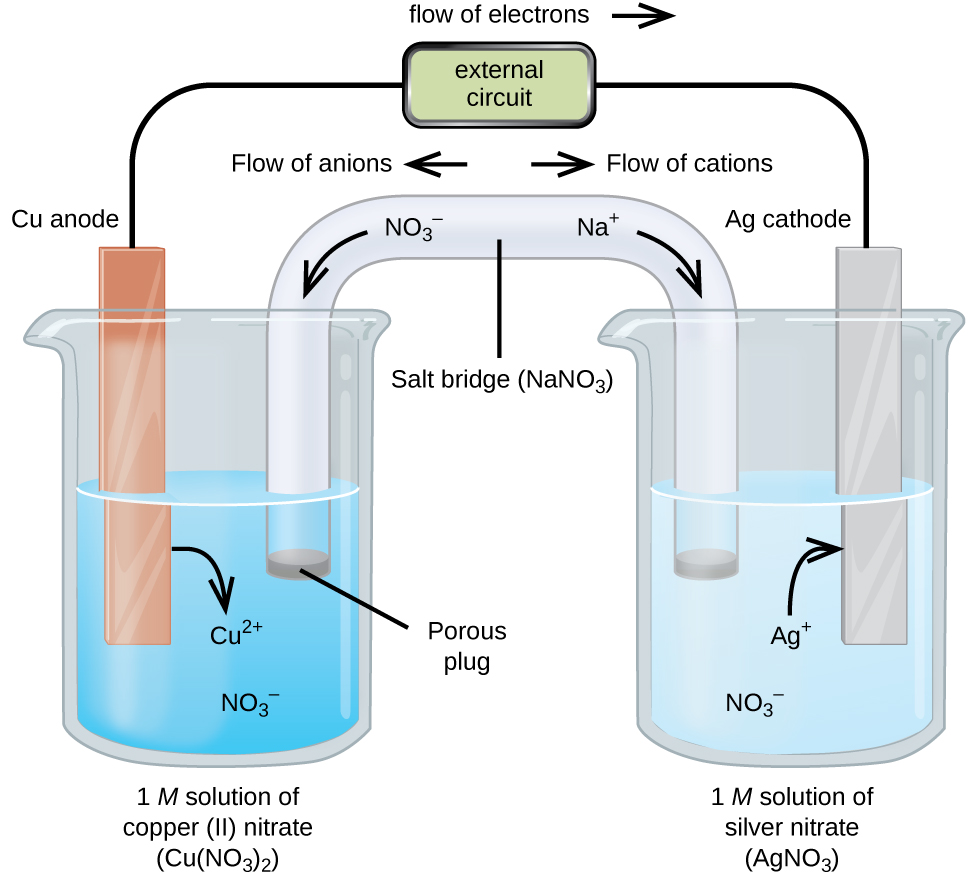
What Is The Working Principle Of Electrochemical Cells . This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. The chemical reaction occuring inside these cells is known as electrolysis. Describe the basic components of electrochemical cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. List some of the characteristics, applications and limitations of cells and batteries.
from alevelchemistry.co.uk
Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Describe the basic components of electrochemical cells. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. The chemical reaction occuring inside these cells is known as electrolysis. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. List some of the characteristics, applications and limitations of cells and batteries.
Electrochemical Cells Definition, Description & Types
What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. List some of the characteristics, applications and limitations of cells and batteries. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. Describe the basic components of electrochemical cells. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. The chemical reaction occuring inside these cells is known as electrolysis. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. The chemical reaction occuring inside these cells is known. What Is The Working Principle Of Electrochemical Cells.
From www.pearson.com
Electrochemical Cells Video Tutorial & Practice Channels for Pearson+ What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Describe the basic components of electrochemical cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell is a device that produces an electric current from energy. What Is The Working Principle Of Electrochemical Cells.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation ID2216847 What Is The Working Principle Of Electrochemical Cells Describe the basic components of electrochemical cells. List some of the characteristics, applications and limitations of cells and batteries. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. An apparatus that is used. What Is The Working Principle Of Electrochemical Cells.
From www.researchgate.net
Schematic representation of the electrochemical cell used in this What Is The Working Principle Of Electrochemical Cells An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that. What Is The Working Principle Of Electrochemical Cells.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle What Is The Working Principle Of Electrochemical Cells Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. List some of the characteristics, applications and limitations of cells and batteries. The chemical reaction occuring inside these cells is known as electrolysis. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrochemical. What Is The Working Principle Of Electrochemical Cells.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General What Is The Working Principle Of Electrochemical Cells Describe the basic components of electrochemical cells. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. What Is The Working Principle Of Electrochemical Cells.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. The chemical reaction occuring inside these cells is known as electrolysis. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Describe the basic components of electrochemical cells. List some. What Is The Working Principle Of Electrochemical Cells.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses What Is The Working Principle Of Electrochemical Cells Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. Describe the basic components of electrochemical cells. List some of the characteristics, applications and limitations of cells and batteries. An apparatus that is used. What Is The Working Principle Of Electrochemical Cells.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is The Working Principle Of Electrochemical Cells An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. The chemical reaction occuring inside these cells is known as electrolysis. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. List some of the characteristics, applications and limitations of. What Is The Working Principle Of Electrochemical Cells.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 10 simple voltaic cell What Is The Working Principle Of Electrochemical Cells An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Describe the basic components of electrochemical cells. The chemical reaction occuring inside these cells is known as electrolysis. Electrochemical cells,. What Is The Working Principle Of Electrochemical Cells.
From saylordotorg.github.io
Electrochemistry What Is The Working Principle Of Electrochemical Cells An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. Electrochemical cells, also known as galvanic cells. What Is The Working Principle Of Electrochemical Cells.
From schoolbag.info
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Describe the basic components of electrochemical cells. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An apparatus that is. What Is The Working Principle Of Electrochemical Cells.
From classfullbooklice.z13.web.core.windows.net
Working Of Electrochemical Cell What Is The Working Principle Of Electrochemical Cells Describe the basic components of electrochemical cells. The chemical reaction occuring inside these cells is known as electrolysis. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. Electrochemical cells, also known as galvanic cells or. What Is The Working Principle Of Electrochemical Cells.
From www.pinterest.com
Galvanic Cell or Voltaic cell Construction and working What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An apparatus that is used to generate electricity from a spontaneous. What Is The Working Principle Of Electrochemical Cells.
From saylordotorg.github.io
Describing Electrochemical Cells What Is The Working Principle Of Electrochemical Cells An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. Electrolytic. What Is The Working Principle Of Electrochemical Cells.
From greenenergymaterial.com
Basics Of Electrochemical Cells » Green Energy Material What Is The Working Principle Of Electrochemical Cells Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. List some of the characteristics, applications and limitations of cells and batteries. An electrochemical cell is a device that converts chemical energy. What Is The Working Principle Of Electrochemical Cells.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is The Working Principle Of Electrochemical Cells Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. List some of the characteristics, applications and limitations of cells and batteries. Describe the basic components of electrochemical cells. An apparatus that is used. What Is The Working Principle Of Electrochemical Cells.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field What Is The Working Principle Of Electrochemical Cells This kind of cell includes the galvanic, or voltaic, cell, named after luigi. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. Describe the basic components of electrochemical cells. An apparatus. What Is The Working Principle Of Electrochemical Cells.
From gaskatel.de
Principles of electrochemical cells Gaskatel What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. List some of the characteristics, applications and limitations of cells and batteries. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell is a device that produces. What Is The Working Principle Of Electrochemical Cells.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram What Is The Working Principle Of Electrochemical Cells This kind of cell includes the galvanic, or voltaic, cell, named after luigi. List some of the characteristics, applications and limitations of cells and batteries. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an. What Is The Working Principle Of Electrochemical Cells.
From www.youtube.com
Class 12 How electricity is generated in an electrochemical cell YouTube What Is The Working Principle Of Electrochemical Cells An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. Electrolytic cell is a type of electrochemical cell that. What Is The Working Principle Of Electrochemical Cells.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An electrochemical cell is a device that converts chemical energy into. What Is The Working Principle Of Electrochemical Cells.
From saylordotorg.github.io
Electrochemistry What Is The Working Principle Of Electrochemical Cells The chemical reaction occuring inside these cells is known as electrolysis. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. List some of the characteristics, applications and limitations of cells and batteries. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an. What Is The Working Principle Of Electrochemical Cells.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 What Is The Working Principle Of Electrochemical Cells Describe the basic components of electrochemical cells. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. The chemical reaction occuring inside these cells is known as electrolysis. List some of the characteristics, applications and limitations of cells and batteries. Electrolytic cell is a type of electrochemical cell that uses electric. What Is The Working Principle Of Electrochemical Cells.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii What Is The Working Principle Of Electrochemical Cells This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. List some of the characteristics, applications and limitations of cells and batteries. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that. What Is The Working Principle Of Electrochemical Cells.
From 2012books.lardbucket.org
Electrochemistry What Is The Working Principle Of Electrochemical Cells This kind of cell includes the galvanic, or voltaic, cell, named after luigi. The chemical reaction occuring inside these cells is known as electrolysis. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions.. What Is The Working Principle Of Electrochemical Cells.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from. What Is The Working Principle Of Electrochemical Cells.
From present5.com
Foundation Year Program Electrochemistry Lecture A Principles of What Is The Working Principle Of Electrochemical Cells An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. Electrolytic cell is a type of electrochemical cell that uses electric currents to speed up cell reactions. Describe the basic components of electrochemical cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. What Is The Working Principle Of Electrochemical Cells.
From theedge.com.hk
Chemistry How To Electrochemical Cell The Edge What Is The Working Principle Of Electrochemical Cells An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. Describe the basic components of electrochemical cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a.. What Is The Working Principle Of Electrochemical Cells.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID What Is The Working Principle Of Electrochemical Cells Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. The chemical reaction occuring inside these cells is known as electrolysis. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous. What Is The Working Principle Of Electrochemical Cells.
From knowledgecycle.in
‘Electrochemical Cell’ Chemistry Investigatory Project PDF » Knowledge What Is The Working Principle Of Electrochemical Cells An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. List some of the characteristics, applications and limitations of cells and batteries. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. This kind of cell includes the galvanic, or voltaic, cell,. What Is The Working Principle Of Electrochemical Cells.
From www.thoughtco.com
Electrochemical Cell Definition What Is The Working Principle Of Electrochemical Cells List some of the characteristics, applications and limitations of cells and batteries. An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that. What Is The Working Principle Of Electrochemical Cells.
From chem.libretexts.org
11.4 Voltammetric Methods Chemistry LibreTexts What Is The Working Principle Of Electrochemical Cells This kind of cell includes the galvanic, or voltaic, cell, named after luigi. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. List some of the characteristics, applications and limitations of cells and batteries. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy. What Is The Working Principle Of Electrochemical Cells.
From www.studocu.com
3. Construction and working of an ZnCu electrochemical cell What Is The Working Principle Of Electrochemical Cells An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions involving an anode,. Describe the basic components of electrochemical cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. List some of the characteristics, applications and limitations of cells and batteries. An apparatus that. What Is The Working Principle Of Electrochemical Cells.
From www.youtube.com
The operation of a lightemitting electrochemical cell YouTube What Is The Working Principle Of Electrochemical Cells List some of the characteristics, applications and limitations of cells and batteries. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrolytic cell is a type of electrochemical cell. What Is The Working Principle Of Electrochemical Cells.