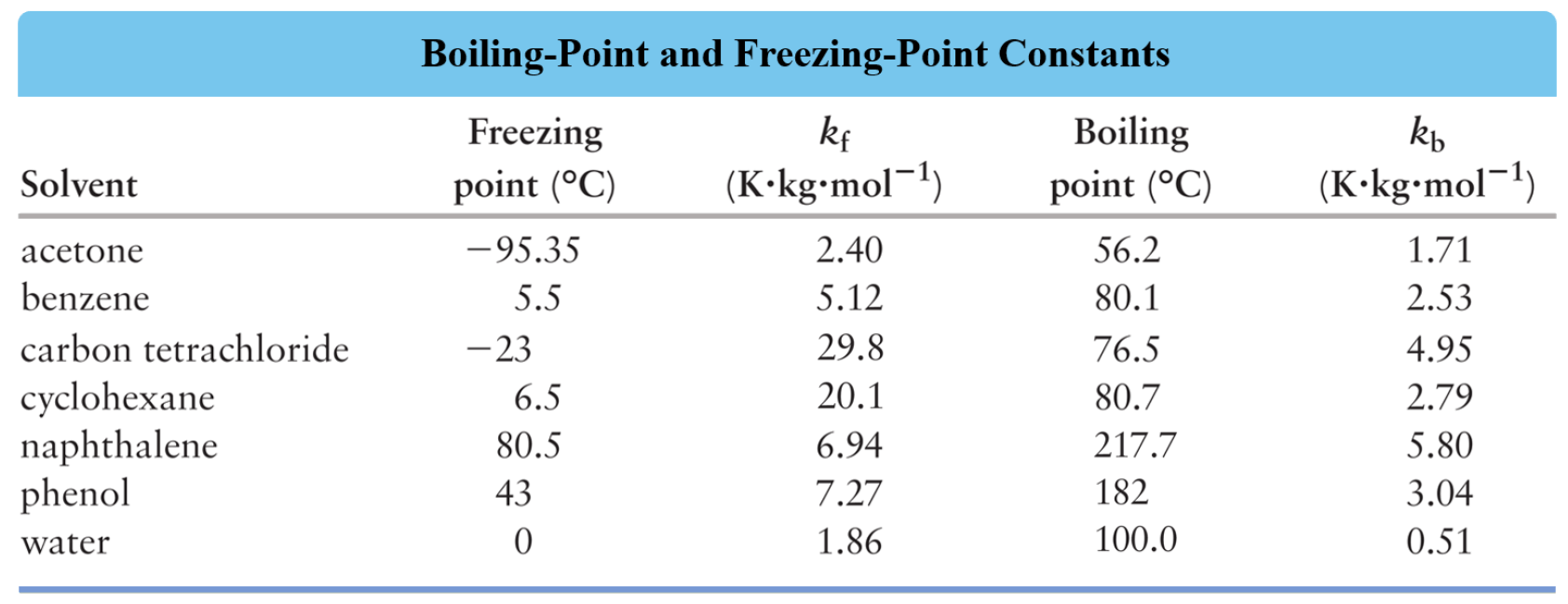
Which Solvent Has The Highest Boiling Point . Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). The solvent with the highest boiling point is. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. The solvent with the highest. It also shows that the boiling points of alcohols. This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). This is true for any. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Find the boiling points of various solvents at standard pressure for your laboratory work.
from general.chemistrysteps.com
In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). The solvent with the highest. This is true for any. Find the boiling points of various solvents at standard pressure for your laboratory work. The solvent with the highest boiling point is. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. It also shows that the boiling points of alcohols. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight.
Boiling Point Elevation Chemistry Steps
Which Solvent Has The Highest Boiling Point 47 rows find the boiling point and other properties of various solvents used in organic chemistry. This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. Find the boiling points of various solvents at standard pressure for your laboratory work. The solvent with the highest. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). It also shows that the boiling points of alcohols. This is true for any. The solvent with the highest boiling point is.
From www.toppr.com
Which of the following aqueous solution will have the highest boiling Which Solvent Has The Highest Boiling Point It also shows that the boiling points of alcohols. The solvent with the highest boiling point is. This is true for any. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). 47 rows find the boiling point and other properties of various solvents used in organic chemistry. Solvent name density. Which Solvent Has The Highest Boiling Point.
From www.numerade.com
SOLVED Arrange the boiling points of the aqueous solutions relative to Which Solvent Has The Highest Boiling Point Find the boiling points of various solvents at standard pressure for your laboratory work. This is true for any. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. The solvent with the highest. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). The. Which Solvent Has The Highest Boiling Point.
From www.chegg.com
Solved 13 pts Question 3 Which of the following compounds Which Solvent Has The Highest Boiling Point The solvent with the highest. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. The solvent with the highest boiling point is. Find the boiling points of various solvents at standard pressure. Which Solvent Has The Highest Boiling Point.
From www.numerade.com
SOLVED Arrange the compounds from lowest boiling point to highest Which Solvent Has The Highest Boiling Point Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). The solvent with the highest boiling point is. This table shows that alcohols (in blue) have higher boiling points than haloalkanes. Which Solvent Has The Highest Boiling Point.
From www.chegg.com
Solved a) Create a Series containing the boiling points of Which Solvent Has The Highest Boiling Point Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular. Which Solvent Has The Highest Boiling Point.
From www.numerade.com
SOLVED Constants for freezingpoint depression and boilingpoint Which Solvent Has The Highest Boiling Point Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). The solvent with the highest boiling point is. The solvent with the highest. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. Find the boiling points of various solvents at standard pressure for. Which Solvent Has The Highest Boiling Point.
From kunduz.com
[ANSWERED] D 57 180 Aqueous solution of which has highest boiling point Which Solvent Has The Highest Boiling Point The solvent with the highest. This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. This is true for any. The solvent with the highest boiling point is. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). 47 rows. Which Solvent Has The Highest Boiling Point.
From questions-in.kunduz.com
The boiling point of a solution is higher... Physical Chemistry Which Solvent Has The Highest Boiling Point The solvent with the highest boiling point is. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). 47 rows find the boiling point and other properties of various solvents used in organic chemistry. The solvent with the highest. This is true for any. The boiling point elevation (\(δt_b\)) and freezing. Which Solvent Has The Highest Boiling Point.
From www.chegg.com
Solved Which substance has the highest boiling point? Select Which Solvent Has The Highest Boiling Point This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). Find the boiling points of various solvents at standard pressure for your laboratory work. In order for the saltwater solution. Which Solvent Has The Highest Boiling Point.
From www.slideserve.com
PPT Entry Task Nov 27 th Block 1 PowerPoint Presentation, free Which Solvent Has The Highest Boiling Point This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). This is true for any.. Which Solvent Has The Highest Boiling Point.
From www.numerade.com
SOLVED Question 16 (3 points) The compound with the highest boiling Which Solvent Has The Highest Boiling Point The solvent with the highest boiling point is. This is true for any. This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). The solvent with the highest. It also. Which Solvent Has The Highest Boiling Point.
From www.toppr.com
Which of the following aqueous solution has the highest freezing point? Which Solvent Has The Highest Boiling Point The solvent with the highest. Find the boiling points of various solvents at standard pressure for your laboratory work. It also shows that the boiling points of alcohols. The solvent with the highest boiling point is. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). 47 rows find the. Which Solvent Has The Highest Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Which Solvent Has The Highest Boiling Point The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. It also shows that the boiling points of alcohols. Find the boiling points of various solvents at standard pressure for your laboratory work. The solvent with the highest boiling point is. This is true for any. This table shows. Which Solvent Has The Highest Boiling Point.
From www.researchgate.net
Extraction at boiling point of solvents Download Scientific Diagram Which Solvent Has The Highest Boiling Point It also shows that the boiling points of alcohols. The solvent with the highest boiling point is. The solvent with the highest. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. This. Which Solvent Has The Highest Boiling Point.
From www.numerade.com
SOLVED Identify the compound with the highest boiling point Which Solvent Has The Highest Boiling Point The solvent with the highest boiling point is. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). This is true for any. It also shows that the boiling points of alcohols. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences. Which Solvent Has The Highest Boiling Point.
From www.researchgate.net
Melting Point, Boiling Point, and Heat of Vaporization of Some Common Which Solvent Has The Highest Boiling Point Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). The solvent with the highest. The solvent with the highest boiling point is. This is true for any. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. In. Which Solvent Has The Highest Boiling Point.
From www.bartleby.com
Answered Which solution has the highest boiling… bartleby Which Solvent Has The Highest Boiling Point The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). Find the boiling points of various solvents at standard pressure for your laboratory work. This is true for any.. Which Solvent Has The Highest Boiling Point.
From www.numerade.com
SOLVED You have just made a new compound in the lab and need to choose Which Solvent Has The Highest Boiling Point Find the boiling points of various solvents at standard pressure for your laboratory work. It also shows that the boiling points of alcohols. The solvent with the highest. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). 47 rows find the boiling point and other properties of various solvents used. Which Solvent Has The Highest Boiling Point.
From www.youtube.com
Which Compound Has a Higher Boiling Point? Intermolecular Force Boiling Which Solvent Has The Highest Boiling Point 47 rows find the boiling point and other properties of various solvents used in organic chemistry. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). This table shows. Which Solvent Has The Highest Boiling Point.
From askfilo.com
Which of the following aqueous solution has the highest boiling point? (A.. Which Solvent Has The Highest Boiling Point The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. This is true for any. The solvent with the highest boiling point is. It also shows that the boiling points of alcohols. The solvent with the highest. 47 rows find the boiling point and other properties of various solvents. Which Solvent Has The Highest Boiling Point.
From www.bartleby.com
Answered Choose the aqueous solution that has… bartleby Which Solvent Has The Highest Boiling Point 47 rows find the boiling point and other properties of various solvents used in organic chemistry. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). It also shows that the boiling points of alcohols. The solvent with the highest boiling point is. The solvent with the highest. This is. Which Solvent Has The Highest Boiling Point.
From www.chegg.com
Solved Which substance has the highest boiling point? Which Solvent Has The Highest Boiling Point The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Find the boiling points of various solvents at standard pressure for your laboratory work. This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. The solvent with the highest.. Which Solvent Has The Highest Boiling Point.
From www.chegg.com
Solved 25) Which of the following has the highest boiling Which Solvent Has The Highest Boiling Point Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). Find the boiling points of various solvents at standard pressure for your laboratory work. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). It also shows that the boiling points. Which Solvent Has The Highest Boiling Point.
From www.coursehero.com
[Solved] Which compound has the higher boiling point? O CH3CH3 O Which Solvent Has The Highest Boiling Point In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. Solvent name density boiling melting. Which Solvent Has The Highest Boiling Point.
From brainly.com
Which of the following solutions has the highest boiling point? Which Which Solvent Has The Highest Boiling Point 47 rows find the boiling point and other properties of various solvents used in organic chemistry. It also shows that the boiling points of alcohols. The solvent with the highest. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. In order for the saltwater solution to boil, the. Which Solvent Has The Highest Boiling Point.
From www.coursehero.com
[Solved] Which of these compounds will have the highest boiling point Which Solvent Has The Highest Boiling Point The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight.. Which Solvent Has The Highest Boiling Point.
From www.toppr.com
Which of the following has the highest boiling point? (A) 1 M glucose Which Solvent Has The Highest Boiling Point The solvent with the highest boiling point is. This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the equivalent molecular weight. This is true for any. Find the boiling points of various solvents at standard pressure for your laboratory work. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution. Which Solvent Has The Highest Boiling Point.
From www.youtube.com
(Quiz No. 4) Which Of following aqueous solution should have the Which Solvent Has The Highest Boiling Point It also shows that the boiling points of alcohols. The solvent with the highest boiling point is. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). The solvent with the highest. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. Solvent name density. Which Solvent Has The Highest Boiling Point.
From wou.edu
CH105 Chapter 9 Organic Compounds of Oxygen Chemistry Which Solvent Has The Highest Boiling Point The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Find the boiling points of various solvents at standard pressure for your laboratory work. The solvent with the highest. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. It also shows that. Which Solvent Has The Highest Boiling Point.
From www.slideserve.com
PPT Solvents, Recrystallization, and Melting Point PowerPoint Which Solvent Has The Highest Boiling Point The solvent with the highest boiling point is. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). 47 rows find the boiling point and other properties of various solvents used in organic chemistry. This is true for any. The solvent with the highest. Solvent name density boiling melting flash viscosity. Which Solvent Has The Highest Boiling Point.
From www.youtube.com
Which of the following aqueous solution has highest boiling point? (1 Which Solvent Has The Highest Boiling Point This is true for any. It also shows that the boiling points of alcohols. In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Solvent name density boiling melting flash. Which Solvent Has The Highest Boiling Point.
From www.researchgate.net
Loss tangent values in the order of increasing boiling points for Which Solvent Has The Highest Boiling Point In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). The solvent with the highest boiling point is. Find the boiling points of various solvents at standard pressure for your laboratory work. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between. Which Solvent Has The Highest Boiling Point.
From byjus.com
Which of the following have highest boiling point? (i) 0.1m NaCl (ii) 0 Which Solvent Has The Highest Boiling Point In order for the saltwater solution to boil, the temperature must be raised about \ (100^\text {o} \text {c}\). The solvent with the highest boiling point is. It also shows that the boiling points of alcohols. This is true for any. Find the boiling points of various solvents at standard pressure for your laboratory work. Solvent name density boiling melting. Which Solvent Has The Highest Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Which Solvent Has The Highest Boiling Point The solvent with the highest boiling point is. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. This table shows that alcohols (in blue) have higher boiling points. Which Solvent Has The Highest Boiling Point.
From www.microcare.com
Does The Boiling Point of a Solvent Affect Results MicroCare Which Solvent Has The Highest Boiling Point The solvent with the highest boiling point is. Solvent name density boiling melting flash viscosity dielectric uv refractive point point point (cp, 20 ºc) constant cutoffindex (ºc) (ºc). It also shows that the boiling points of alcohols. 47 rows find the boiling point and other properties of various solvents used in organic chemistry. The solvent with the highest. The boiling. Which Solvent Has The Highest Boiling Point.