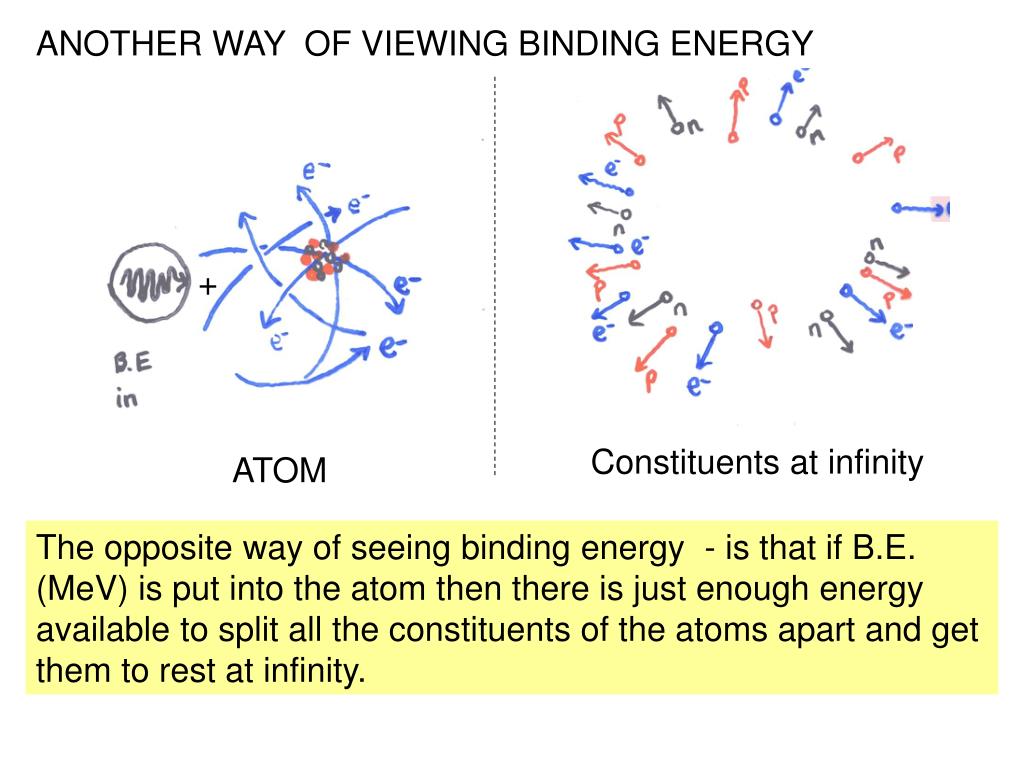
Binding Energy And Kinetic Energy . Calculate mass defect and binding energy for nuclei. Use a graph of binding energy per nucleon (ben) versus mass number. Explain trends in the relative stability of nuclei. It is also equal to the difference between the sum of the binding. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Calculate the binding energy per nucleon of a particle. Here, binding energy is the energy of an electron attracted to a nucleus; Define and discuss binding energy. This energy derives from the. Calculate the mass defect and binding energy for a wide range of nuclei; 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). The more tightly bound a system is, the stronger the. Atoms present in compound being tested by xps are determined according to the equation: The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model.
from www.slideserve.com
Calculate mass defect and binding energy for nuclei. Define and discuss binding energy. Use a graph of binding energy per nucleon (ben) versus mass number. This energy derives from the. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). Here, binding energy is the energy of an electron attracted to a nucleus; Nuclear chemistry is the study of. Atoms present in compound being tested by xps are determined according to the equation:
PPT Binding energy in atoms and nuclei PowerPoint Presentation, free
Binding Energy And Kinetic Energy Explain trends in the relative stability of nuclei. It is also equal to the difference between the sum of the binding. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Calculate the mass defect and binding energy for a wide range of nuclei; Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). Nuclear chemistry is the study of. Define and discuss binding energy. Use a graph of binding energy per nucleon (ben) versus mass number. Atoms present in compound being tested by xps are determined according to the equation: Explain trends in the relative stability of nuclei. Calculate mass defect and binding energy for nuclei. The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. Calculate the binding energy per nucleon of a particle. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. The more tightly bound a system is, the stronger the. This energy derives from the.
From byjus.com
Why Fe has highest binding energy and the binding energy curve Binding Energy And Kinetic Energy Use a graph of binding energy per nucleon (ben) versus mass number. Calculate the binding energy per nucleon of a particle. Calculate mass defect and binding energy for nuclei. This energy derives from the. Define and discuss binding energy. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. It is. Binding Energy And Kinetic Energy.
From www.researchgate.net
Binding energy BE, matter radius r, and energy T of 3 He with Binding Energy And Kinetic Energy 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Calculate the binding energy per nucleon of a particle. The more tightly bound a system is, the stronger the. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The. Binding Energy And Kinetic Energy.
From www.researchgate.net
XPS spectra showing the binding energy shifts of (a) W, (b) N, (c) O Binding Energy And Kinetic Energy Calculate the binding energy per nucleon of a particle. The more tightly bound a system is, the stronger the. Calculate mass defect and binding energy for nuclei. Define and discuss binding energy. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Binding things together lowers the total energy. Binding Energy And Kinetic Energy.
From courses.lumenlearning.com
The Photoelectric Effect Physics Binding Energy And Kinetic Energy Explain trends in the relative stability of nuclei. Here, binding energy is the energy of an electron attracted to a nucleus; Calculate mass defect and binding energy for nuclei. Define and discuss binding energy. Atoms present in compound being tested by xps are determined according to the equation: This energy derives from the. The more tightly bound a system is,. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT SOME EXAMPLES PowerPoint Presentation, free download ID3574214 Binding Energy And Kinetic Energy Here, binding energy is the energy of an electron attracted to a nucleus; 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The mass difference between the parent and. Binding Energy And Kinetic Energy.
From www.toppr.com
Magnitude of binding energy of satellite is E and energy is K Binding Energy And Kinetic Energy Nuclear chemistry is the study of. The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. Define and discuss binding energy. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Calculate the binding energy per nucleon of a. Binding Energy And Kinetic Energy.
From www.tessshebaylo.com
Binding Energy Equation Tessshebaylo Binding Energy And Kinetic Energy Explain trends in the relative stability of nuclei. Define and discuss binding energy. The more tightly bound a system is, the stronger the. It is also equal to the difference between the sum of the binding. Calculate mass defect and binding energy for nuclei. Here, binding energy is the energy of an electron attracted to a nucleus; Calculate the binding. Binding Energy And Kinetic Energy.
From owlcation.com
How to Understand Energy, Momentum and Work Done Owlcation Binding Energy And Kinetic Energy Define and discuss binding energy. Nuclear chemistry is the study of. This energy derives from the. The more tightly bound a system is, the stronger the. Atoms present in compound being tested by xps are determined according to the equation: Explain trends in the relative stability of nuclei. Calculate mass defect and binding energy for nuclei. The binding energy (be). Binding Energy And Kinetic Energy.
From eduinput.com
What is Binding Energy?Definition, Types, And Applications Binding Energy And Kinetic Energy Explain trends in the relative stability of nuclei. It is also equal to the difference between the sum of the binding. Calculate mass defect and binding energy for nuclei. Calculate the mass defect and binding energy for a wide range of nuclei; Define and discuss binding energy. The more tightly bound a system is, the stronger the. Binding things together. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT Binding energy in atoms and nuclei PowerPoint Presentation, free Binding Energy And Kinetic Energy The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. Calculate mass defect and binding energy for nuclei. Explain trends in the relative stability of nuclei. Here, binding energy is the energy of an electron attracted to a nucleus; Calculate the binding energy per nucleon of a particle. Use a. Binding Energy And Kinetic Energy.
From www.researchgate.net
Schematic representation of bindingenergy curves and probability Binding Energy And Kinetic Energy Explain trends in the relative stability of nuclei. The more tightly bound a system is, the stronger the. Nuclear chemistry is the study of. Calculate the mass defect and binding energy for a wide range of nuclei; The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. This energy derives. Binding Energy And Kinetic Energy.
From www.researchgate.net
Molecular orbitals, electron binding energy and energy for Binding Energy And Kinetic Energy Here, binding energy is the energy of an electron attracted to a nucleus; Calculate the mass defect and binding energy for a wide range of nuclei; This energy derives from the. Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). Calculate mass defect and binding energy for nuclei. The binding energy (be) of. Binding Energy And Kinetic Energy.
From www.etutorworld.com
and Potential Energy Definitions, Key Difference, Examples and Binding Energy And Kinetic Energy Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Here, binding energy. Binding Energy And Kinetic Energy.
From www.youtube.com
Understand Potential Binding Energy Curve Physics Inter Atomic Binding Energy And Kinetic Energy Explain trends in the relative stability of nuclei. Calculate the mass defect and binding energy for a wide range of nuclei; The more tightly bound a system is, the stronger the. Calculate the binding energy per nucleon of a particle. Use a graph of binding energy per nucleon (ben) versus mass number. It is also equal to the difference between. Binding Energy And Kinetic Energy.
From curiophysics.com
Binding Energy Per Nucleon Binding Energy Curve » Curio Physics Binding Energy And Kinetic Energy The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. Explain trends in the relative stability of nuclei. Define and discuss binding energy. Here, binding energy is the energy of an electron attracted to a nucleus; 7 rows it addresses the mass and kinetic energy of the parts that bind. Binding Energy And Kinetic Energy.
From general.chemistrysteps.com
Nuclear Binding Energy Chemistry Steps Binding Energy And Kinetic Energy The more tightly bound a system is, the stronger the. Calculate the mass defect and binding energy for a wide range of nuclei; Nuclear chemistry is the study of. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. 7 rows it addresses the mass and kinetic energy of the parts. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT Binding energy in atoms and nuclei PowerPoint Presentation, free Binding Energy And Kinetic Energy It is also equal to the difference between the sum of the binding. The more tightly bound a system is, the stronger the. Explain trends in the relative stability of nuclei. Use a graph of binding energy per nucleon (ben) versus mass number. Calculate the mass defect and binding energy for a wide range of nuclei; Binding things together lowers. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT Introduction PowerPoint Presentation, free download ID6535249 Binding Energy And Kinetic Energy 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. It is also equal to the difference between the sum of the binding. Calculate the binding energy per nucleon of a particle. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT Binding Energy PowerPoint Presentation, free download ID1150105 Binding Energy And Kinetic Energy Calculate mass defect and binding energy for nuclei. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Calculate the binding energy per nucleon of a particle. Nuclear chemistry is the study of. The binding energy (be) of a nucleus is the energy needed to separate it into individual. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT Physics of Radiography Chapter 4 PowerPoint Presentation, free Binding Energy And Kinetic Energy Here, binding energy is the energy of an electron attracted to a nucleus; Define and discuss binding energy. Calculate the mass defect and binding energy for a wide range of nuclei; Explain trends in the relative stability of nuclei. It is also equal to the difference between the sum of the binding. This energy derives from the. Atoms present in. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT Nuclear notation and Binding energy Contents Atomic notation Binding Energy And Kinetic Energy Explain trends in the relative stability of nuclei. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). The more tightly bound a system is, the stronger the. Here, binding energy is the. Binding Energy And Kinetic Energy.
From general.chemistrysteps.com
Photoelectric Effect Chemistry Steps Binding Energy And Kinetic Energy Calculate mass defect and binding energy for nuclei. Here, binding energy is the energy of an electron attracted to a nucleus; Explain trends in the relative stability of nuclei. Atoms present in compound being tested by xps are determined according to the equation: The more tightly bound a system is, the stronger the. The mass difference between the parent and. Binding Energy And Kinetic Energy.
From www.universetoday.com
What is Binding Energy? Universe Today Binding Energy And Kinetic Energy Calculate mass defect and binding energy for nuclei. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Atoms present in compound being tested by xps are determined according to the equation: The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT Binding Energy Binding Energy per Nucleon PowerPoint Presentation Binding Energy And Kinetic Energy Calculate mass defect and binding energy for nuclei. Define and discuss binding energy. Calculate the mass defect and binding energy for a wide range of nuclei; Calculate the binding energy per nucleon of a particle. Atoms present in compound being tested by xps are determined according to the equation: 7 rows it addresses the mass and kinetic energy of the. Binding Energy And Kinetic Energy.
From www.youtube.com
Binding energy YouTube Binding Energy And Kinetic Energy Here, binding energy is the energy of an electron attracted to a nucleus; It is also equal to the difference between the sum of the binding. Define and discuss binding energy. This energy derives from the. Calculate the binding energy per nucleon of a particle. Nuclear chemistry is the study of. Binding things together lowers the total energy in the. Binding Energy And Kinetic Energy.
From www.pinterest.com.mx
Understanding Physics, Learn Physics, Basic Physics, How To Study Binding Energy And Kinetic Energy The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. Use a graph of binding energy per nucleon (ben) versus mass number. Explain trends in the relative stability of nuclei. Calculate the mass defect and binding energy for a wide range of nuclei; Calculate mass defect and binding energy for. Binding Energy And Kinetic Energy.
From www.energyencyclopedia.com
Binding energy curve Images Free Downloads Energy Encyclopedia Binding Energy And Kinetic Energy Calculate mass defect and binding energy for nuclei. The more tightly bound a system is, the stronger the. It is also equal to the difference between the sum of the binding. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Nuclear chemistry is the study of. Calculate the. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT CHAPTER 25 Nuclear Chemistry PowerPoint Presentation, free Binding Energy And Kinetic Energy Use a graph of binding energy per nucleon (ben) versus mass number. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Define and discuss binding energy. Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). The binding energy (be) of a. Binding Energy And Kinetic Energy.
From www.tessshebaylo.com
Binding Energy Equation Physics Tessshebaylo Binding Energy And Kinetic Energy Nuclear chemistry is the study of. Use a graph of binding energy per nucleon (ben) versus mass number. 7 rows it addresses the mass and kinetic energy of the parts that bind the various quarks together inside a hadron. Calculate mass defect and binding energy for nuclei. It is also equal to the difference between the sum of the binding.. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT Binding energy in atoms and nuclei PowerPoint Presentation, free Binding Energy And Kinetic Energy Explain trends in the relative stability of nuclei. Calculate the binding energy per nucleon of a particle. It is also equal to the difference between the sum of the binding. Nuclear chemistry is the study of. The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. The more tightly bound. Binding Energy And Kinetic Energy.
From www.youtube.com
CHEM 101 Photoelectric Effect Threshold Frequency and Binding Energy Binding Energy And Kinetic Energy Nuclear chemistry is the study of. Calculate the binding energy per nucleon of a particle. Calculate the mass defect and binding energy for a wide range of nuclei; Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). Explain trends in the relative stability of nuclei. Define and discuss binding energy. Calculate mass defect. Binding Energy And Kinetic Energy.
From www.youtube.com
What is binding energy of a satellite? YouTube Binding Energy And Kinetic Energy Nuclear chemistry is the study of. The more tightly bound a system is, the stronger the. Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). Define and discuss binding energy. Calculate mass defect and binding energy for nuclei. Explain trends in the relative stability of nuclei. The mass difference between the parent and. Binding Energy And Kinetic Energy.
From eduinput.com
EnergyDefinition,Types,And Work Energy Principle in Term of Binding Energy And Kinetic Energy This energy derives from the. Calculate the binding energy per nucleon of a particle. Define and discuss binding energy. The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. Calculate the mass defect and binding energy for a wide range of nuclei; Use a graph of binding energy per nucleon. Binding Energy And Kinetic Energy.
From www.slideserve.com
PPT How to Get the Binding Energy by Mass Spectrometry PowerPoint Binding Energy And Kinetic Energy Calculate the binding energy per nucleon of a particle. Binding things together lowers the total energy in the system (neglecting kinetic energy for the moment). Calculate mass defect and binding energy for nuclei. Define and discuss binding energy. Explain trends in the relative stability of nuclei. It is also equal to the difference between the sum of the binding. The. Binding Energy And Kinetic Energy.
From www.researchgate.net
He I UPS spectra at the low energy region (a) and the Binding Energy And Kinetic Energy Nuclear chemistry is the study of. Calculate the mass defect and binding energy for a wide range of nuclei; Atoms present in compound being tested by xps are determined according to the equation: This energy derives from the. The mass difference between the parent and daughter nucleus can usually be estimated quite well from the liquid drop model. Calculate mass. Binding Energy And Kinetic Energy.