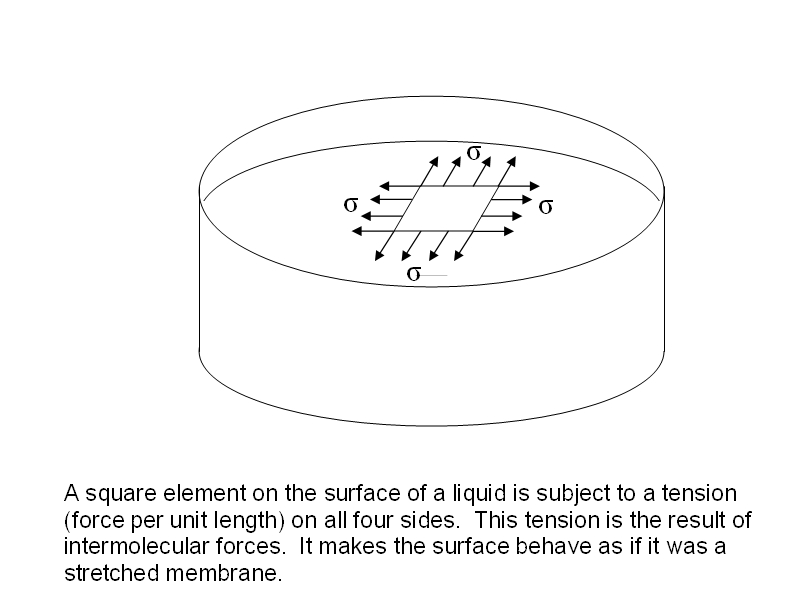
How To Compare Surface Tension . Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. How could we compare the surface tension of water and alcohol? Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension can explain cohesive and adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the. Cohesive forces hold the liquid body with minimum surface area. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or.
from fsz.ifas.ufl.edu
Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension can explain cohesive and adhesive forces. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is the energy required to increase the surface area of a liquid by a given amount. How could we compare the surface tension of water and alcohol? Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces hold the liquid body with minimum surface area. The stronger the intermolecular interactions, the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
Surface Tension
How To Compare Surface Tension Equivalently, it can be stated as surface energy in ergs per square centimeter. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces hold the liquid body with minimum surface area. The stronger the intermolecular interactions, the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension can explain cohesive and adhesive forces. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. How could we compare the surface tension of water and alcohol?
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How To Compare Surface Tension Cohesive forces hold the liquid body with minimum surface area. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. How could we compare the surface tension of water and alcohol? Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The. How To Compare Surface Tension.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How To Compare Surface Tension Cohesive forces hold the liquid body with minimum surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. The stronger the intermolecular interactions, the. Surface tension can explain cohesive and. How To Compare Surface Tension.
From fsz.ifas.ufl.edu
Surface Tension How To Compare Surface Tension Equivalently, it can be stated as surface energy in ergs per square centimeter. How could we compare the surface tension of water and alcohol? Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film. How To Compare Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How To Compare Surface Tension Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension can explain cohesive and adhesive forces. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling. How To Compare Surface Tension.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector How To Compare Surface Tension Surface tension can explain cohesive and adhesive forces. Cohesive forces hold the liquid body with minimum surface area. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Equivalently, it can be stated as. How To Compare Surface Tension.
From brainly.in
The dimensional formula for the surface tension? Brainly.in How To Compare Surface Tension Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a given amount. How could. How To Compare Surface Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How To Compare Surface Tension How could we compare the surface tension of water and alcohol? Cohesive forces hold the liquid body with minimum surface area. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Equivalently, it can be stated as surface energy in ergs per square centimeter. The stronger the intermolecular interactions, the.. How To Compare Surface Tension.
From www.youtube.com
Surface Tension of Water Explained YouTube How To Compare Surface Tension Surface tension can explain cohesive and adhesive forces. How could we compare the surface tension of water and alcohol? Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the. How To Compare Surface Tension.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and How To Compare Surface Tension Equivalently, it can be stated as surface energy in ergs per square centimeter. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. How could we compare the surface tension. How To Compare Surface Tension.
From funsizephysics.com
What is Surface Tension? FunsizePhysics How To Compare Surface Tension How could we compare the surface tension of water and alcohol? Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Equivalently, it can be stated as surface energy in ergs per. How To Compare Surface Tension.
From tfiglobalnews.com
The Relation Between Surface Tension and Surface Energy TFIGlobal How To Compare Surface Tension The stronger the intermolecular interactions, the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is. How To Compare Surface Tension.
From owlcation.com
Surfactant Lowering Pulmonary Surface Tension Owlcation How To Compare Surface Tension Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. How could we compare the surface tension of water and alcohol? Cohesive forces hold the liquid body with minimum surface area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Students. How To Compare Surface Tension.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson How To Compare Surface Tension Surface tension can explain cohesive and adhesive forces. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces hold the liquid body with minimum surface area. How could we compare. How To Compare Surface Tension.
From www.biolinscientific.com
3 ways to measure surface tension How To Compare Surface Tension Surface tension can explain cohesive and adhesive forces. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Cohesive forces hold the liquid body with minimum surface area. The stronger the intermolecular interactions, the. How could we compare the surface tension of water and alcohol? Cohesive forces between molecules cause. How To Compare Surface Tension.
From www.adda247.com
Surface Tension Definition, Formula, Examples How To Compare Surface Tension How could we compare the surface tension of water and alcohol? Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film. How To Compare Surface Tension.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download How To Compare Surface Tension Surface tension is the energy required to increase the surface area of a liquid by a given amount. Equivalently, it can be stated as surface energy in ergs per square centimeter. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Cohesive forces between molecules cause the surface of a. How To Compare Surface Tension.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How To Compare Surface Tension Equivalently, it can be stated as surface energy in ergs per square centimeter. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. How could we compare the surface tension of water and alcohol? Surface tension can explain cohesive and adhesive forces. The stronger the intermolecular interactions, the. Surface tension is the. How To Compare Surface Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How To Compare Surface Tension The stronger the intermolecular interactions, the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test. How To Compare Surface Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How To Compare Surface Tension Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. How could we compare the surface tension of water and alcohol? The stronger the intermolecular interactions, the. Surface tension can explain cohesive and adhesive forces. Equivalently, it can be stated as surface energy in ergs per square centimeter. Cohesive forces. How To Compare Surface Tension.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube How To Compare Surface Tension Cohesive forces hold the liquid body with minimum surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Students may suggest placing an equal number of drops of each liquid on wax. How To Compare Surface Tension.
From www.youtube.com
Surface tension Pressure difference across curved surfaces (Laplace How To Compare Surface Tension Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. How could we compare the surface tension of water and alcohol?. How To Compare Surface Tension.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query How To Compare Surface Tension The stronger the intermolecular interactions, the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces hold the liquid body with minimum surface area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension can explain cohesive and. How To Compare Surface Tension.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement How To Compare Surface Tension How could we compare the surface tension of water and alcohol? Cohesive forces hold the liquid body with minimum surface area. The stronger the intermolecular interactions, the. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Cohesive forces between molecules cause the surface of a liquid to contract to. How To Compare Surface Tension.
From www.youtube.com
SURFACE TENSION & INTERFACIAL PHENOMENON PART1 INTERFACE TYPES How To Compare Surface Tension Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension can explain cohesive and adhesive forces. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Surface tension is typically measured in dynes/cm, the force in dynes required to break. How To Compare Surface Tension.
From www.researchgate.net
Schematic illustration of standard methods of surface tension How To Compare Surface Tension The stronger the intermolecular interactions, the. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension can explain cohesive and adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required. How To Compare Surface Tension.
From byjus.com
Explain the surface tension phenomenon with examples. How To Compare Surface Tension Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension can explain cohesive and adhesive forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface. How To Compare Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How To Compare Surface Tension Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. How could we compare the surface tension of water and alcohol? The stronger the intermolecular interactions, the. Surface tension is. How To Compare Surface Tension.
From fphoto.photoshelter.com
science chemistry surface tension Fundamental Photographs The Art How To Compare Surface Tension Surface tension can explain cohesive and adhesive forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. How could we compare the surface tension of water and alcohol? Cohesive forces hold. How To Compare Surface Tension.
From www.researchgate.net
Schematic illustration of standard methods of surface tension How To Compare Surface Tension Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension can explain cohesive and adhesive forces. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. The stronger the intermolecular interactions, the. Students may suggest placing an equal number of. How To Compare Surface Tension.
From pressbooks.nscc.ca
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action How To Compare Surface Tension How could we compare the surface tension of water and alcohol? Cohesive forces hold the liquid body with minimum surface area. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension can explain cohesive and adhesive forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to. How To Compare Surface Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How To Compare Surface Tension Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the. Students may suggest placing an equal number of drops of each liquid on wax. How To Compare Surface Tension.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences How To Compare Surface Tension Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Cohesive forces hold the liquid body with minimum surface area. Surface tension can explain cohesive and adhesive forces. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is the energy required to increase the. How To Compare Surface Tension.
From mungfali.com
Surface Tension Chart How To Compare Surface Tension Cohesive forces hold the liquid body with minimum surface area. Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How could we compare the surface tension of water and. How To Compare Surface Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How To Compare Surface Tension Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension can explain cohesive. How To Compare Surface Tension.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How To Compare Surface Tension Cohesive forces hold the liquid body with minimum surface area. How could we compare the surface tension of water and alcohol? Students may suggest placing an equal number of drops of each liquid on wax paper, overfilling a test tube, or. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. How To Compare Surface Tension.