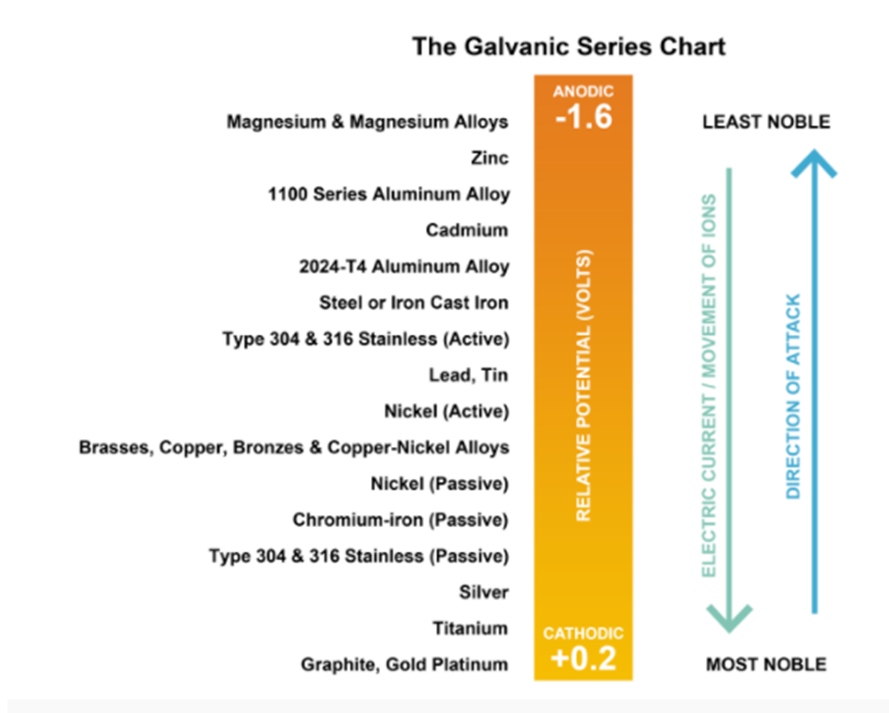
How To Prevent Galvanic Corrosion Between Aluminum And Titanium . To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. Breaking the electrical connection by insulating. To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. Galvanic corrosion can be prevented by: Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. Selecting materials with similar corrosion potentials. Stainless steel (active) + aluminum. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. Learn the causes of galvanic corrosion and get tips on how to prevent or minimize the corrosion when designing and manufacturing sheet metal parts. Stainless steel acts as the cathode, and aluminum acts as an anode. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s.
from www.iccons.com.au
For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. Galvanic corrosion can be prevented by: To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. Learn the causes of galvanic corrosion and get tips on how to prevent or minimize the corrosion when designing and manufacturing sheet metal parts. Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. Breaking the electrical connection by insulating. Stainless steel acts as the cathode, and aluminum acts as an anode.
The Insight Galvanic Corrosion and Fasteners ICCONS
How To Prevent Galvanic Corrosion Between Aluminum And Titanium Galvanic corrosion can be prevented by: To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: Stainless steel acts as the cathode, and aluminum acts as an anode. Breaking the electrical connection by insulating. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. Selecting materials with similar corrosion potentials. Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. Stainless steel (active) + aluminum. Galvanic corrosion can be prevented by: To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. Learn the causes of galvanic corrosion and get tips on how to prevent or minimize the corrosion when designing and manufacturing sheet metal parts. In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time.
From www.langleyalloys.it
How to prevent galvanic corrosion between aluminium and brass Langley How To Prevent Galvanic Corrosion Between Aluminum And Titanium To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. Breaking the electrical connection by insulating. Stainless steel acts as the cathode, and aluminum acts as an anode. Stainless steel (active) + aluminum. In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.simpletwig.com
Galvanic Action Corrosion Prevention Architect's Blog How To Prevent Galvanic Corrosion Between Aluminum And Titanium In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. In. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From gluethings.com
How can we prevent galvanic corrosion between aluminum and brass How To Prevent Galvanic Corrosion Between Aluminum And Titanium Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. Selecting materials with similar corrosion potentials. Stainless steel acts as the cathode, and aluminum acts. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.corrosionpedia.com
5 Ways to Avoid Galvanic Corrosion How To Prevent Galvanic Corrosion Between Aluminum And Titanium Breaking the electrical connection by insulating. Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: Galvanic corrosion can be prevented by: To mitigate the risk of galvanic corrosion, there are certain metals that should not be. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.reddit.com
Is there galvanic corrosion between titanium and aluminum? r/bikewrench How To Prevent Galvanic Corrosion Between Aluminum And Titanium Stainless steel acts as the cathode, and aluminum acts as an anode. In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. To mitigate the risk of galvanic corrosion,. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From gluethings.com
How can we prevent galvanic corrosion between aluminum and brass How To Prevent Galvanic Corrosion Between Aluminum And Titanium Stainless steel acts as the cathode, and aluminum acts as an anode. Selecting materials with similar corrosion potentials. Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. For this reason, titanium and its. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From branchinvestigations.com
How to Prevent Galvanic Corrosion Branch Property Investigations How To Prevent Galvanic Corrosion Between Aluminum And Titanium Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. Galvanic. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From branchinvestigations.com
How to Prevent Galvanic Corrosion Branch Property Investigations How To Prevent Galvanic Corrosion Between Aluminum And Titanium Selecting materials with similar corrosion potentials. Galvanic corrosion can be prevented by: To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. Stainless steel (active) + aluminum. To. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.museoinclusivo.com
How to Prevent Galvanic Corrosion Between Aluminum and Stainless Steel How To Prevent Galvanic Corrosion Between Aluminum And Titanium Breaking the electrical connection by insulating. In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From eastmarineasia.com
How to Stop ☠ Galvanic Corrosion ☠ East Marine Asia How To Prevent Galvanic Corrosion Between Aluminum And Titanium Learn the causes of galvanic corrosion and get tips on how to prevent or minimize the corrosion when designing and manufacturing sheet metal parts. Selecting materials with similar corrosion potentials. Stainless steel (active) + aluminum. Breaking the electrical connection by insulating. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From metalprofy.com
How to Prevent Galvanic Corrosion Between Aluminum and Steel? MetalProfy How To Prevent Galvanic Corrosion Between Aluminum And Titanium In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. To prevent galvanic corrosion from occurring, one element of the. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.vrogue.co
Your Guide To Treating Galvanic Corrosion vrogue.co How To Prevent Galvanic Corrosion Between Aluminum And Titanium To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. Selecting materials with similar corrosion potentials. Stainless steel (active) + aluminum. Galvanic corrosion can be prevented by: Below, we. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From blog.fabreeka.com
The Science Behind Galvanic Corrosion and How To Prevent It How To Prevent Galvanic Corrosion Between Aluminum And Titanium To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. Stainless steel acts as the cathode, and aluminum acts as an anode. In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.langleyalloys.it
How to prevent galvanic corrosion between aluminium and brass Langley How To Prevent Galvanic Corrosion Between Aluminum And Titanium In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. Stainless steel (active) + aluminum. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. In this post, we’ll provide an overview of the key concepts involved in galvanic. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.iccons.com.au
The Insight Galvanic Corrosion and Fasteners ICCONS How To Prevent Galvanic Corrosion Between Aluminum And Titanium Breaking the electrical connection by insulating. Galvanic corrosion can be prevented by: To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. Selecting materials with. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From mavink.com
Galvanic Corrosion Aluminum Stainless Steel How To Prevent Galvanic Corrosion Between Aluminum And Titanium Stainless steel (active) + aluminum. Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From gluethings.com
How can we prevent galvanic corrosion between aluminum and brass How To Prevent Galvanic Corrosion Between Aluminum And Titanium To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. Galvanic corrosion can be prevented by: Stainless steel (active) + aluminum. In this post, we’ll. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.ifam.fraunhofer.de
How to avoid galvanic corrosion? How To Prevent Galvanic Corrosion Between Aluminum And Titanium Selecting materials with similar corrosion potentials. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. Galvanic corrosion can be prevented by: To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. Learn the causes. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.balkanplumbing.com
Three Ways To Prevent Galvanic Corrosion In Your Pipes How To Prevent Galvanic Corrosion Between Aluminum And Titanium Galvanic corrosion can be prevented by: In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. Below, we have provided a galvanic series corrosion chart showcasing the metal combinations. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.youtube.com
Galvanic Corrosion what causes it and how to prevent, have an issue on How To Prevent Galvanic Corrosion Between Aluminum And Titanium In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. Stainless. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From mavink.com
Galvanic Corrosion Chart Dissimilar Metals How To Prevent Galvanic Corrosion Between Aluminum And Titanium Selecting materials with similar corrosion potentials. Breaking the electrical connection by insulating. Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. In the presence of water or moisture, galvanic corrosion can occur at. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.museoinclusivo.com
How to Prevent Galvanic Corrosion Between Aluminum and Steel Aluminum How To Prevent Galvanic Corrosion Between Aluminum And Titanium For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f) in sour. Stainless steel (active) + aluminum. Galvanic corrosion can be prevented by: To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. In this post, we’ll. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.balkanplumbing.com
Three Ways To Prevent Galvanic Corrosion In Your Pipes How To Prevent Galvanic Corrosion Between Aluminum And Titanium Stainless steel (active) + aluminum. Stainless steel acts as the cathode, and aluminum acts as an anode. Learn the causes of galvanic corrosion and get tips on how to prevent or minimize the corrosion when designing and manufacturing sheet metal parts. In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From chansmachining.com
Overview of Corrosion Resistant Metals and Alloys ChansMachining How To Prevent Galvanic Corrosion Between Aluminum And Titanium To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.museoinclusivo.com
How to Prevent Galvanic Corrosion Between Aluminum and Steel Aluminum How To Prevent Galvanic Corrosion Between Aluminum And Titanium Selecting materials with similar corrosion potentials. To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. Galvanic corrosion can be prevented by: In this post, we’ll provide an overview of the key. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.langleyalloys.it
How to prevent galvanic corrosion between aluminium and brass Langley How To Prevent Galvanic Corrosion Between Aluminum And Titanium In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. Stainless steel acts as the cathode, and aluminum acts as an. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.museoinclusivo.com
How to Prevent Galvanic Corrosion Between Aluminum and Steel Aluminum How To Prevent Galvanic Corrosion Between Aluminum And Titanium Breaking the electrical connection by insulating. Selecting materials with similar corrosion potentials. In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. Learn the causes of galvanic corrosion and get tips on how to prevent or minimize the corrosion when designing and manufacturing sheet metal parts. Unlike. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From makersbolt.com
What is Galvanic Corrosion and How to Prevent It Makers Bolt How To Prevent Galvanic Corrosion Between Aluminum And Titanium Learn the causes of galvanic corrosion and get tips on how to prevent or minimize the corrosion when designing and manufacturing sheet metal parts. To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. Stainless steel acts as the cathode, and aluminum acts as an anode. Galvanic corrosion can be prevented by:. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.slideserve.com
PPT 5 Ways to Avoid Galvanic Corrosion PowerPoint Presentation, free How To Prevent Galvanic Corrosion Between Aluminum And Titanium Selecting materials with similar corrosion potentials. To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From blog.thepipingmart.com
How to Prevent Galvanic Corrosion Between Aluminum and Stainless Steel How To Prevent Galvanic Corrosion Between Aluminum And Titanium To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: Stainless steel (active) + aluminum. In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion and how to mitigate the effects with preventative chromium coating solutions. Breaking the electrical connection by insulating. Learn the causes of galvanic corrosion and get. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From recipepes.com
how to prevent galvanic corrosion between aluminum and steel How To Prevent Galvanic Corrosion Between Aluminum And Titanium Stainless steel (active) + aluminum. Selecting materials with similar corrosion potentials. Learn the causes of galvanic corrosion and get tips on how to prevent or minimize the corrosion when designing and manufacturing sheet metal parts. For this reason, titanium and its alloys must not be coupled with carbon steel, aluminium, zinc or active stainless steels at temperatures above 75°c (167°f). How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From edu.svet.gob.gt
The Science Behind Galvanic Corrosion And How To Prevent It How To Prevent Galvanic Corrosion Between Aluminum And Titanium Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. To mitigate the risk of galvanic corrosion, there are certain metals that should not be used in conjunction. Breaking the electrical connection by insulating. Galvanic corrosion can be prevented by: Learn the causes of galvanic corrosion and get tips on how to. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From www.youtube.com
How To Protect Aluminum From Corrosion Vapor Honing Technologies How To Prevent Galvanic Corrosion Between Aluminum And Titanium Galvanic corrosion can be prevented by: Learn the causes of galvanic corrosion and get tips on how to prevent or minimize the corrosion when designing and manufacturing sheet metal parts. Stainless steel (active) + aluminum. Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. For this reason, titanium and its alloys. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From blog.thepipingmart.com
3 Ways to Prevent Corrosion of Metal Parts How To Prevent Galvanic Corrosion Between Aluminum And Titanium Below, we have provided a galvanic series corrosion chart showcasing the metal combinations that carry the highest risk. Selecting materials with similar corrosion potentials. In the presence of water or moisture, galvanic corrosion can occur at these junctions, compromising the integrity of the plumbing network over time. Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.
From blog.fabreeka.com
The Science Behind Galvanic Corrosion and How To Prevent It How To Prevent Galvanic Corrosion Between Aluminum And Titanium Breaking the electrical connection by insulating. To prevent galvanic corrosion from occurring, one element of the corrosion cell must be interrupted: Galvanic corrosion can be prevented by: Unlike some metals whose oxide layer can be flaky, brittle, and eventually spall off (such as steel), titanium’s. In this post, we’ll provide an overview of the key concepts involved in galvanic corrosion. How To Prevent Galvanic Corrosion Between Aluminum And Titanium.