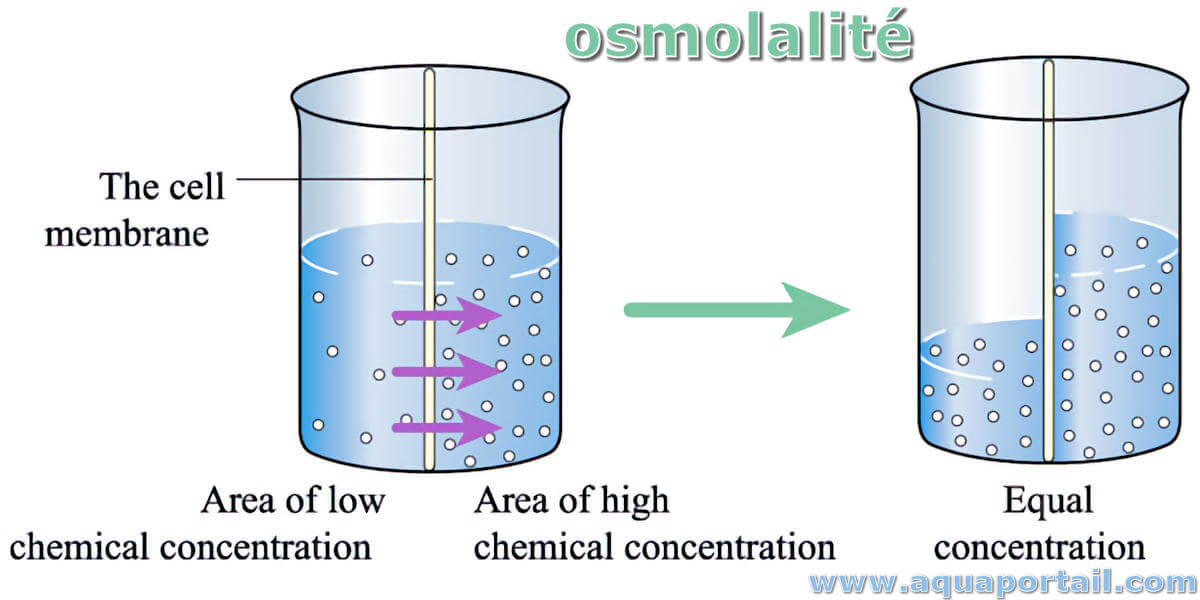
Osmolarity Definition Biology Simple . Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Explain why osmoregulation and osmotic balance are important body functions;. These are consequential values are derived from the na+, k+ ions,. Define osmosis and explain its role within molecules; Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolarity is nearly the same as osmolality.
from www.aquaportail.com
A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. These are consequential values are derived from the na+, k+ ions,. Define osmosis and explain its role within molecules; Osmolarity is nearly the same as osmolality. Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Osmolality is a measure of the concentration of particles in the serum per kilogram of water.
Osmolalité définition et explications
Osmolarity Definition Biology Simple A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Explain why osmoregulation and osmotic balance are important body functions;. These are consequential values are derived from the na+, k+ ions,. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Define osmosis and explain its role within molecules; Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolarity is nearly the same as osmolality.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary Osmolarity Definition Biology Simple A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Explain why osmoregulation and osmotic balance are important body functions;. These are consequential values are derived from the na+, k+ ions,. Define osmosis and explain its role within molecules; Osmolality is a measure of. Osmolarity Definition Biology Simple.
From www.youtube.com
Calculated Osmolality YouTube Osmolarity Definition Biology Simple A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is nearly the same as osmolality. Explain why osmoregulation and osmotic balance are important body functions;. These. Osmolarity Definition Biology Simple.
From www.coastalwiki.org
Osmosis Coastal Wiki Osmolarity Definition Biology Simple A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Osmolarity is nearly the same as osmolality. Osmolarity is basically the degree of how. Osmolarity Definition Biology Simple.
From www.yaclass.in
Osmosis — lesson. Science CBSE, Class 9. Osmolarity Definition Biology Simple Define osmosis and explain its role within molecules; Osmolarity is nearly the same as osmolality. Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. A solution’s molarity is the number of moles of solute per liter of solution, while a. Osmolarity Definition Biology Simple.
From www.scribd.com
Definition Osmolarity PDF Osmolarity Definition Biology Simple Osmolality is a measure of the concentration of particles in the serum per kilogram of water. These are consequential values are derived from the na+, k+ ions,. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolarity is nearly the same as osmolality.. Osmolarity Definition Biology Simple.
From www.slideserve.com
PPT Fluid & Electrolyte Balance PowerPoint Presentation, free Osmolarity Definition Biology Simple Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Define osmosis and explain its role within molecules; Osmolality is a measure of the. Osmolarity Definition Biology Simple.
From www.slideserve.com
PPT Biology STAAR Review PowerPoint Presentation, free download ID Osmolarity Definition Biology Simple Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Osmolarity is nearly the same as osmolality. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Explain why osmoregulation and osmotic balance are. Osmolarity Definition Biology Simple.
From courses.lumenlearning.com
Osmoregulation and Osmotic Balance OpenStax Biology 2e Osmolarity Definition Biology Simple Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Osmolarity is nearly the same as osmolality. Define osmosis and explain its role within molecules; Osmolality is a measure of the concentration of particles in the serum per kilogram of water. A solution’s molarity is the number of moles of solute. Osmolarity Definition Biology Simple.
From www.slideshare.net
Regulation of osmolarity Osmolarity Definition Biology Simple Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Explain why osmoregulation and osmotic balance are important body functions;. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is nearly the same as osmolality. These are consequential values are derived from. Osmolarity Definition Biology Simple.
From study.com
Serum Osmolality Definition, Calculation & Interpretation Lesson Osmolarity Definition Biology Simple Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Explain why osmoregulation and osmotic balance are important body functions;. These are consequential values are derived from the na+, k+ ions,.. Osmolarity Definition Biology Simple.
From www.britannica.com
Cell Membrane Transport, Osmosis, Diffusion Britannica Osmolarity Definition Biology Simple A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Osmolarity is nearly the same as osmolality. Explain why osmoregulation and osmotic balance are. Osmolarity Definition Biology Simple.
From www.pinterest.com
Difference Between Osmolarity and Osmolality Definition, Explanation Osmolarity Definition Biology Simple A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Define osmosis and explain its role within molecules; These are consequential values are derived from the na+, k+ ions,. Osmolarity is a measure of the total concentration of solute particles in a solution, typically. Osmolarity Definition Biology Simple.
From pediaa.com
Difference Between Osmolarity and Tonicity Osmolarity Definition Biology Simple Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of. Osmolarity Definition Biology Simple.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube Osmolarity Definition Biology Simple Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Osmolarity is nearly the same as osmolality. Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. These are consequential values. Osmolarity Definition Biology Simple.
From www.osmosis.org
Osmoregulation Video, Anatomy, Definition & Function Osmosis Osmolarity Definition Biology Simple A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity is nearly the same as osmolality. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in. Osmolarity Definition Biology Simple.
From www.researchgate.net
Osmolarity changes in the lysosome lumen. Cargo macromolecules (white Osmolarity Definition Biology Simple These are consequential values are derived from the na+, k+ ions,. Define osmosis and explain its role within molecules; Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity is nearly the same as osmolality. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. A solution’s molarity is. Osmolarity Definition Biology Simple.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 Osmolarity Definition Biology Simple Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Osmolarity is nearly the same. Osmolarity Definition Biology Simple.
From www.sciencefacts.net
Osmosis Definition and How Does it Occur (with Diagram) Osmolarity Definition Biology Simple Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is nearly the same as osmolality. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. These are consequential values are derived from the na+, k+ ions,. Define osmosis and explain its role within. Osmolarity Definition Biology Simple.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 Osmolarity Definition Biology Simple Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Define osmosis and explain its role within molecules; Osmolarity is nearly the same as osmolality. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. A solution’s molarity is the number. Osmolarity Definition Biology Simple.
From www.yourdictionary.com
Main Difference Between Osmosis and Diffusion in Biology YourDictionary Osmolarity Definition Biology Simple Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number. Osmolarity Definition Biology Simple.
From www.studocu.com
Cell Biology Osmolarity concentration Cell Biology Osmolarity Osmolarity Definition Biology Simple Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Define osmosis and explain its role within molecules; Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. These are consequential values are derived from the na+, k+ ions,. Osmolarity is nearly the same as. Osmolarity Definition Biology Simple.
From www.youtube.com
Osmolarity Example (Bio) YouTube Osmolarity Definition Biology Simple Osmolality is a measure of the concentration of particles in the serum per kilogram of water. These are consequential values are derived from the na+, k+ ions,. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity is nearly. Osmolarity Definition Biology Simple.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis ditki medical Osmolarity Definition Biology Simple Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Define osmosis and explain its role within molecules; Osmolarity is nearly the same as osmolality. Osmolarity is basically the degree of how many solute. Osmolarity Definition Biology Simple.
From sciencenotes.org
Osmotic Pressure Definition, Formula, Examples Osmolarity Definition Biology Simple Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. These are consequential values are derived from the na+, k+ ions,.. Osmolarity Definition Biology Simple.
From ar.inspiredpencil.com
Osmosis Definition Biology Osmolarity Definition Biology Simple Osmolarity is nearly the same as osmolality. Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. These are consequential values are derived from the na+, k+ ions,. Osmolality is a measure of the concentration of particles in the serum per. Osmolarity Definition Biology Simple.
From www.aquaportail.com
Osmolalité définition et explications Osmolarity Definition Biology Simple Osmolarity is nearly the same as osmolality. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Define osmosis and explain its role within molecules; Explain why osmoregulation. Osmolarity Definition Biology Simple.
From drawittoknowit.com
Cell Biology Osmosis and Osmolarity ditki medical & biological sciences Osmolarity Definition Biology Simple Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is nearly the same as osmolality. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Define osmosis and explain its role within molecules; A solution’s molarity is the number of moles of solute. Osmolarity Definition Biology Simple.
From biology.reachingfordreams.com
Functions of the kidney in a human body Parts of the kidney Biology Osmolarity Definition Biology Simple A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Osmolarity is basically the degree of how many solute osmoles are existing in 1. Osmolarity Definition Biology Simple.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry Osmolarity Definition Biology Simple Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. A solution’s molarity is the number of moles of solute per liter of solution,. Osmolarity Definition Biology Simple.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary Osmolarity Definition Biology Simple Osmolality is a measure of the concentration of particles in the serum per kilogram of water. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles. Osmolarity Definition Biology Simple.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download Osmolarity Definition Biology Simple Osmolarity is nearly the same as osmolality. Define osmosis and explain its role within molecules; These are consequential values are derived from the na+, k+ ions,. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Osmolality is a measure of the concentration of particles in the serum per kilogram of. Osmolarity Definition Biology Simple.
From www.slideserve.com
PPT IV Therapy PowerPoint Presentation, free download ID9628818 Osmolarity Definition Biology Simple Explain why osmoregulation and osmotic balance are important body functions;. These are consequential values are derived from the na+, k+ ions,. A solution’s molarity is the number of moles of solute per liter of solution, while a solution’s molality is the number of moles of solute per. Osmolarity is nearly the same as osmolality. Osmolarity is basically the degree of. Osmolarity Definition Biology Simple.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 2. Osmolarity ditki Osmolarity Definition Biology Simple Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Define osmosis and explain its role within molecules; These are consequential values are derived from the na+, k+ ions,. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. A solution’s. Osmolarity Definition Biology Simple.
From www.tldrpharmacy.com
Osmolarity vs. Osmolality Because You've Probably the Osmolarity Definition Biology Simple Osmolarity is a measure of the total concentration of solute particles in a solution, typically expressed in osmoles per liter. Define osmosis and explain its role within molecules; Osmolality is a measure of the concentration of particles in the serum per kilogram of water. A solution’s molarity is the number of moles of solute per liter of solution, while a. Osmolarity Definition Biology Simple.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary Osmolarity Definition Biology Simple Define osmosis and explain its role within molecules; Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is basically the degree of how many solute osmoles are existing in 1 liter of a standard solution. Explain why osmoregulation and osmotic balance are important body functions;. A solution’s molarity is the number of. Osmolarity Definition Biology Simple.