Titration Lab Ap Chemistry . a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Strong base naoh while recording the ph. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl.
from mungfali.com
what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. Strong base naoh while recording the ph. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard.
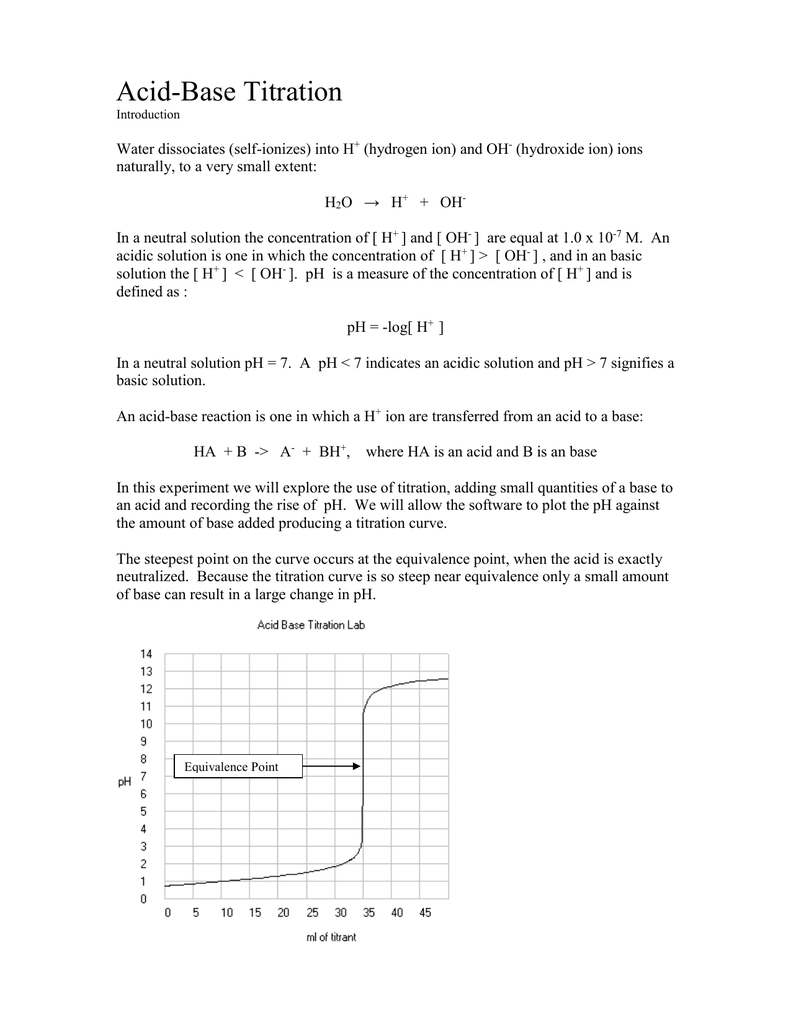
Acid Base Titration Lab
Titration Lab Ap Chemistry To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. Strong base naoh while recording the ph. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard.
From www.youtube.com
Titration Lab AP Chem YouTube Titration Lab Ap Chemistry a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. titration is a method of volumetric analysis —. Titration Lab Ap Chemistry.
From www.studypool.com
SOLUTION 8 5 acid base titrations ap chem Studypool Titration Lab Ap Chemistry Strong base naoh while recording the ph. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. a titration is. Titration Lab Ap Chemistry.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Lab Ap Chemistry To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. Strong base naoh while recording the ph. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. what i absolutely have to know to survive the ap exam. Titration Lab Ap Chemistry.
From www.youtube.com
AP Chemistry Titration Graph problem worksheet review YouTube Titration Lab Ap Chemistry what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. To find how much of naoh it takes to properly titrate and neutralize 10.0. Titration Lab Ap Chemistry.
From www.youtube.com
AP Chem Titration Part 1 YouTube Titration Lab Ap Chemistry what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is a method of volumetric analysis — the use of volume measurements to analyze. Titration Lab Ap Chemistry.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Titration Lab Ap Chemistry what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Strong base naoh while recording the ph. a titration is used to find the concentration. Titration Lab Ap Chemistry.
From apchemlabs.weebly.com
Titration Lab AP Chem Labs Titration Lab Ap Chemistry To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution. Titration Lab Ap Chemistry.
From studylib.net
1 Classic Titration Lab AP Chemistry Titration is a method used to Titration Lab Ap Chemistry a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. Strong base naoh while recording the ph. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. what i absolutely have to know to. Titration Lab Ap Chemistry.
From stephaniesapchemlabs.weebly.com
Titration Lab Stephanie's Wonderful World of AP Chem Labs Titration Lab Ap Chemistry what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. To find how much of naoh it takes to properly titrate and neutralize 10.0. Titration Lab Ap Chemistry.
From yamiletsapchemistrylabs.weebly.com
Titration Lab Yamilet's AP Chemistry Labs Titration Lab Ap Chemistry what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. Strong base naoh while recording the ph. To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. a titration is used to find the concentration of a particular. Titration Lab Ap Chemistry.
From studylib.net
AP Chemistry Titration Virtual Lab Titration Lab Ap Chemistry a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is used to find the concentration of a particular solution with an unknown concentration by. Titration Lab Ap Chemistry.
From studylib.net
WHS • AP Chemistry Lab AcidBase Titrations Lab Group Student 1 Titration Lab Ap Chemistry a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is a method of volumetric analysis — the use of volume measurements. Titration Lab Ap Chemistry.
From mungfali.com
Acid Base Titration Lab Titration Lab Ap Chemistry what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. a titration is a laboratory process used to determine the. Titration Lab Ap Chemistry.
From www.youtube.com
AP Chemistry Notes 8.12 Titration Basics YouTube Titration Lab Ap Chemistry a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. To find how much of naoh it takes to properly titrate. Titration Lab Ap Chemistry.
From www.youtube.com
AP Chem Titration Part 2 YouTube Titration Lab Ap Chemistry a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Strong base naoh while recording the ph. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. what i absolutely have to know. Titration Lab Ap Chemistry.
From mavink.com
Acid Base Titration Lab Procedure Titration Lab Ap Chemistry a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. To find how much of naoh it takes to properly titrate and neutralize 10.0 ml. Titration Lab Ap Chemistry.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Lab Ap Chemistry Strong base naoh while recording the ph. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. To find how much of naoh it takes to properly titrate. Titration Lab Ap Chemistry.
From www.youtube.com
AP Chem unit 8.5 Titration Curves YouTube Titration Lab Ap Chemistry a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. what i absolutely have to know to survive the ap exam the following might indicate the question. Titration Lab Ap Chemistry.
From www.flinnsci.ca
FlinnPREP™ Inquiry Labs for AP® Chemistry AcidBase Titrations Flinn Titration Lab Ap Chemistry a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is a laboratory process used to determine the. Titration Lab Ap Chemistry.
From www.carolina.com
Indicators, pH, and Titrations Kit for AP® Chemistry Titration Lab Ap Chemistry titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting. Titration Lab Ap Chemistry.
From slideplayer.com
AcidBase Titrations AP Chemistry. ppt download Titration Lab Ap Chemistry what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is used to find the concentration of a particular solution with. Titration Lab Ap Chemistry.
From mattpulcine.blogspot.com
Matt's PreAP Chemistry Blog Acids and Bases Titration Lab 2 Titration Lab Ap Chemistry titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. Strong base naoh while recording the ph. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is a laboratory process used to determine the volume of. Titration Lab Ap Chemistry.
From studylib.net
Acid/Base Titration Lab Titration Lab Ap Chemistry a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. a titration is a laboratory process used to determine the. Titration Lab Ap Chemistry.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific Titration Lab Ap Chemistry To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. what i absolutely have to know to survive the ap exam the following might indicate the. Titration Lab Ap Chemistry.
From www.concordiahanoi.org
AP Chemistry Titration Experiment Post Concordia International Titration Lab Ap Chemistry titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. Strong base naoh while recording the ph. a titration is used to find the concentration of a. Titration Lab Ap Chemistry.
From pdfslide.net
(PPT) Acid Base Titrations AP Chemistry Chapter 15. Titration Titration Lab Ap Chemistry To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. titration is a method of volumetric analysis — the use of volume measurements to analyze an. Titration Lab Ap Chemistry.
From www.youtube.com
AP Chemistry Lab 9 Acid Base Titrations YouTube Titration Lab Ap Chemistry To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Strong base naoh while recording the ph. titration is a method of volumetric analysis — the use of. Titration Lab Ap Chemistry.
From ctdvdimri.blogspot.com
Chemistry Honors Blog Lab 14 Titration Lab Titration Lab Ap Chemistry a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is an analytical procedure used to determine the accurate concentration of a. Titration Lab Ap Chemistry.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Lab Ap Chemistry what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. Strong base naoh while recording the ph. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is an analytical procedure used to determine the accurate concentration. Titration Lab Ap Chemistry.
From yamiletsapchemistrylabs.weebly.com
Titration Lab Yamilet's AP Chemistry Labs Titration Lab Ap Chemistry a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. what i absolutely have to know to survive the ap exam the following. Titration Lab Ap Chemistry.
From blog.moravianacademy.org
Students Explore Calorimetry, Titrations in Chemistry Labs Titration Lab Ap Chemistry Strong base naoh while recording the ph. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is used to find. Titration Lab Ap Chemistry.
From www.studocu.com
Titration AP Chem Lab Titration Purpose To use titration Titration Lab Ap Chemistry titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. what i absolutely have to know to survive the ap exam the following might indicate the question deals with buffers and/or. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with. Titration Lab Ap Chemistry.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Lab Ap Chemistry a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. titration is a method of volumetric analysis — the use of volume measurements. Titration Lab Ap Chemistry.
From yamiletsapchemistrylabs.weebly.com
Titration Lab Yamilet's AP Chemistry Labs Titration Lab Ap Chemistry Strong base naoh while recording the ph. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. To find how much of naoh it takes to properly titrate and neutralize 10.0 ml of 1.5 m hcl. a titration is a laboratory process used to determine the volume. Titration Lab Ap Chemistry.
From yamiletsapchemistrylabs.weebly.com
Titration Lab Yamilet's AP Chemistry Labs Titration Lab Ap Chemistry a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of a known concentration. a titration is an analytical procedure used to. Titration Lab Ap Chemistry.