What Is The Valence Shell Electron Configuration Of Noble Gases . the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. All have an ns 2.
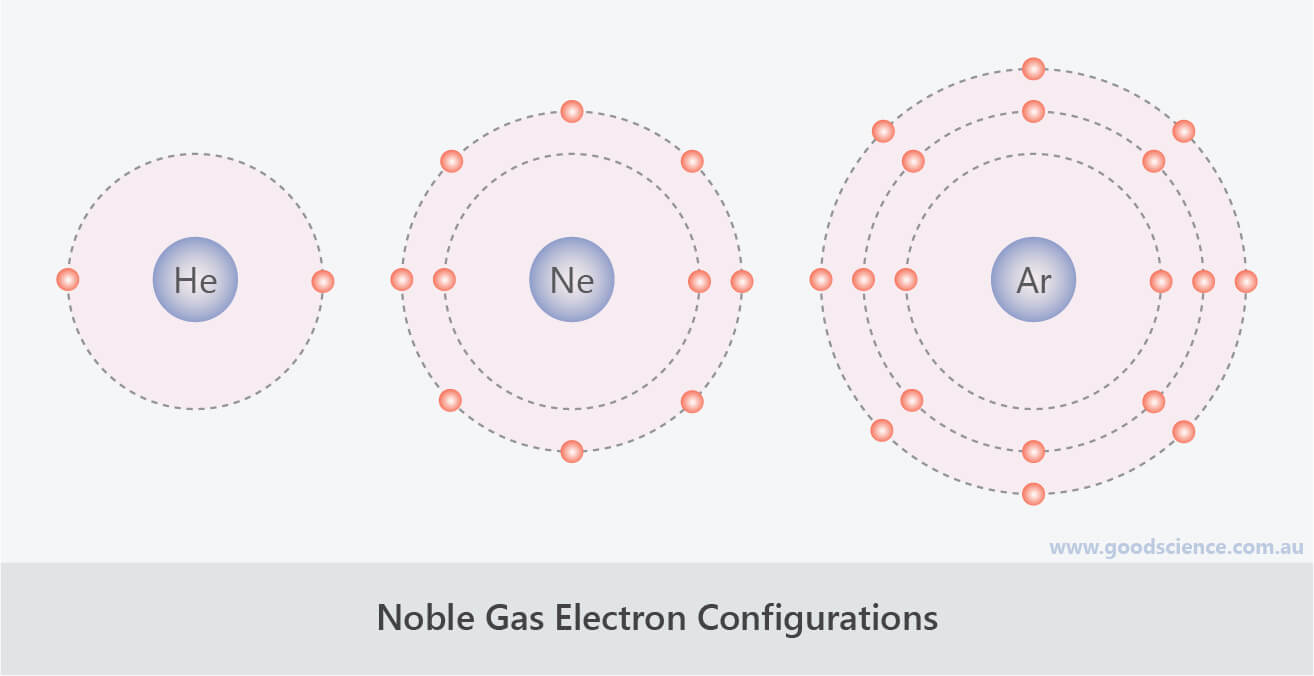
from www.goodscience.com.au
for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. All have an ns 2. the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the.
Formation of Ions and Ionic Compounds Good Science
What Is The Valence Shell Electron Configuration Of Noble Gases for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. All have an ns 2. for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any.
From mungfali.com
Noble Gas Electron Configuration Chart What Is The Valence Shell Electron Configuration Of Noble Gases a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. for transition metals, the number of valence electrons is the number of electrons. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element What Is The Valence Shell Electron Configuration Of Noble Gases use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. All have an ns 2. the electron configuration can be visualized as the core electrons, equivalent to the. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram What Is The Valence Shell Electron Configuration Of Noble Gases the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. a noble gas configuration of an atom consists of the elemental symbol of the last noble. What Is The Valence Shell Electron Configuration Of Noble Gases.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Is The Valence Shell Electron Configuration Of Noble Gases the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. All have an ns 2. for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. use the periodic table to predict the. What Is The Valence Shell Electron Configuration Of Noble Gases.
From animalia-life.club
Noble Gases Electron Configuration What Is The Valence Shell Electron Configuration Of Noble Gases a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. All have an ns 2. the noble gas configuration gives the noble gas. What Is The Valence Shell Electron Configuration Of Noble Gases.
From sciencenotes.org
What Are Noble Gases? Definition and Properties What Is The Valence Shell Electron Configuration Of Noble Gases read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. use the periodic table to predict the characteristic valence electron configuration. What Is The Valence Shell Electron Configuration Of Noble Gases.
From chemnotcheem.com
Covalent bond in molecules O Level Chemistry Notes What Is The Valence Shell Electron Configuration Of Noble Gases All have an ns 2. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. the noble gas configuration gives the noble gas core that occurs before the element. What Is The Valence Shell Electron Configuration Of Noble Gases.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is The Valence Shell Electron Configuration Of Noble Gases use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. All have an ns 2. a noble gas configuration of an atom consists of the elemental symbol of. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is The Valence Shell Electron Configuration Of Noble Gases All have an ns 2. the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. a noble gas configuration of an atom. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.anyrgb.com
Noble Gas, energy Level, atomic Mass, Valence electron, Electron shell What Is The Valence Shell Electron Configuration Of Noble Gases All have an ns 2. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. the electron configuration can be visualized as the. What Is The Valence Shell Electron Configuration Of Noble Gases.
From opentextbc.ca
6.4 Electronic Structure of Atoms (Electron Configurations) Chemistry What Is The Valence Shell Electron Configuration Of Noble Gases use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period,. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.pinterest.com.mx
Electron Configuration Chart for the Elements Chemistry lessons What Is The Valence Shell Electron Configuration Of Noble Gases All have an ns 2. the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. read on to find out what an. What Is The Valence Shell Electron Configuration Of Noble Gases.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is The Valence Shell Electron Configuration Of Noble Gases read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. for transition metals, the number of valence electrons is the number. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.youtube.com
Noble Gas Electron Configurations YouTube What Is The Valence Shell Electron Configuration Of Noble Gases for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. the electron configuration can be visualized as the core electrons, equivalent to the. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.youtube.com
Noble Gas Electron Configurations YouTube What Is The Valence Shell Electron Configuration Of Noble Gases a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. use the periodic table to predict the characteristic valence electron configuration of the. What Is The Valence Shell Electron Configuration Of Noble Gases.
From sciencestruck.com
Everything You Need to Know About Noble Gas Configuration What Is The Valence Shell Electron Configuration Of Noble Gases read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. use the periodic table to predict the characteristic valence electron configuration of the halogens. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.youtube.com
Electron Configuration With Noble Gas Notation YouTube What Is The Valence Shell Electron Configuration Of Noble Gases the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. the noble gas configuration gives the noble gas core that occurs before the element. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.showme.com
Noble Gas Electron Configurations Electron Configuration, High School What Is The Valence Shell Electron Configuration Of Noble Gases the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. read on to find out what an electron configuration is, what valence electrons. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element What Is The Valence Shell Electron Configuration Of Noble Gases a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. for transition metals, the number of valence electrons is the number of electrons. What Is The Valence Shell Electron Configuration Of Noble Gases.
From brainly.in
Write valence shell electronic configuration of the following groups What Is The Valence Shell Electron Configuration Of Noble Gases read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. All have an ns 2. the noble gas configuration gives the noble gas core that occurs before the. What Is The Valence Shell Electron Configuration Of Noble Gases.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry What Is The Valence Shell Electron Configuration Of Noble Gases for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. read on to find out what an electron configuration is, what valence electrons are, and how to find valence. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.sliderbase.com
Valence Electrons Presentation Chemistry What Is The Valence Shell Electron Configuration Of Noble Gases the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. the electron configuration can be visualized as the core electrons, equivalent to. What Is The Valence Shell Electron Configuration Of Noble Gases.
From spmchemistry.blog.onlinetuition.com.my
Stability of Noble Gases SPM Chemistry What Is The Valence Shell Electron Configuration Of Noble Gases use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. the noble gas configuration gives the noble gas core that occurs before the element on the periodic. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary What Is The Valence Shell Electron Configuration Of Noble Gases for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. the noble gas configuration gives the noble gas core that occurs before the element on. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.technologyuk.net
Chemical Bonding What Is The Valence Shell Electron Configuration Of Noble Gases the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. All have an ns 2. for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. a noble gas configuration of an atom. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science What Is The Valence Shell Electron Configuration Of Noble Gases All have an ns 2. read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. for transition metals, the number of valence electrons is the number of electrons. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.slideshare.net
Structure Of Atoms Part 3 What Is The Valence Shell Electron Configuration Of Noble Gases All have an ns 2. for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. the electron configuration can be visualized as the core. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.youtube.com
Electrons in Atoms Lesson7 Noble Gas Configuration YouTube What Is The Valence Shell Electron Configuration Of Noble Gases read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. for transition metals, the number of valence electrons is the number of electrons in. What Is The Valence Shell Electron Configuration Of Noble Gases.
From owlcation.com
What's so Noble About Noble Gases? Owlcation What Is The Valence Shell Electron Configuration Of Noble Gases the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. the noble gas configuration gives the noble gas core that occurs before the. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.youtube.com
Electron Configuration and Noble Gas Core Notation YouTube What Is The Valence Shell Electron Configuration Of Noble Gases the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. for transition metals, the number of valence electrons is the number of electrons in subshells past. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.youtube.com
Valence Electrons & Electron Configurations YouTube What Is The Valence Shell Electron Configuration Of Noble Gases use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. the electron configuration can be visualized as the core electrons, equivalent to the noble gas of the. What Is The Valence Shell Electron Configuration Of Noble Gases.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is The Valence Shell Electron Configuration Of Noble Gases for transition metals, the number of valence electrons is the number of electrons in subshells past the atom’s noble gas core. All have an ns 2. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. read on to find out what an electron. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element What Is The Valence Shell Electron Configuration Of Noble Gases All have an ns 2. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. the noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of. the electron configuration can be visualized as the core electrons, equivalent. What Is The Valence Shell Electron Configuration Of Noble Gases.
From www.anyrgb.com
Noble Gas, niels Bohr, Valence electron, Electron shell, Electron What Is The Valence Shell Electron Configuration Of Noble Gases read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. All have an ns 2. use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. for transition metals, the number of valence electrons is the number of electrons. What Is The Valence Shell Electron Configuration Of Noble Gases.
From sciencenotes.org
List of Electron Configurations of Elements What Is The Valence Shell Electron Configuration Of Noble Gases read on to find out what an electron configuration is, what valence electrons are, and how to find valence electrons of any. a noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by. All have an ns 2. use the periodic table to predict the. What Is The Valence Shell Electron Configuration Of Noble Gases.