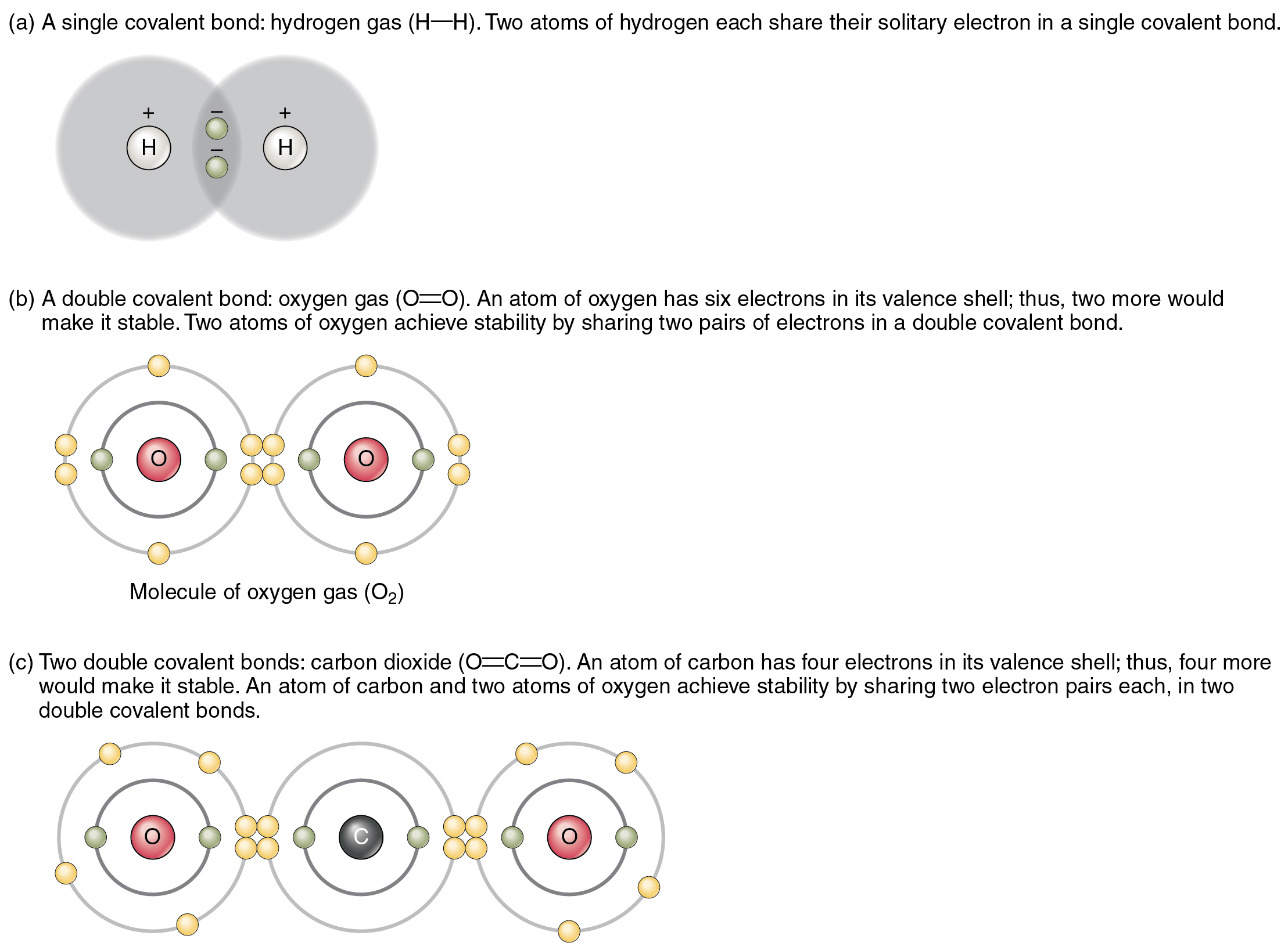
What Is The Electrons Are Shared Between Two Atoms . Usually, sharing electrons gives each atom a full valence shell and. When electrons are shared between two atoms, they make a bond called a covalent bond. An atom that shares one or more of its electrons will complete its outer. The electrons involved are in the outer shells of the atoms. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. Let us illustrate a covalent bond by using. It determines how the shared.
from philschatz.com
Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Usually, sharing electrons gives each atom a full valence shell and. An atom that shares one or more of its electrons will complete its outer. It determines how the shared. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Let us illustrate a covalent bond by using. The electrons involved are in the outer shells of the atoms. When electrons are shared between two atoms, they make a bond called a covalent bond.
Chemical Bonds · Anatomy and Physiology
What Is The Electrons Are Shared Between Two Atoms Let us illustrate a covalent bond by using. Usually, sharing electrons gives each atom a full valence shell and. The electrons involved are in the outer shells of the atoms. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. It determines how the shared. Let us illustrate a covalent bond by using. An atom that shares one or more of its electrons will complete its outer. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. When electrons are shared between two atoms, they make a bond called a covalent bond. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond.
From byjus.com
The total number of electrons shared between the atoms of a hydrogen What Is The Electrons Are Shared Between Two Atoms The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Usually, sharing electrons gives each atom a full valence shell and. It determines how the shared. An atom that shares one or more of its electrons will complete its outer. Let us illustrate a covalent bond by using. When electrons. What Is The Electrons Are Shared Between Two Atoms.
From emedia.rmit.edu.au
Covalent bonds Learning Lab What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. The sharing of a pair of electrons represents a single covalent bond,. What Is The Electrons Are Shared Between Two Atoms.
From www.slideserve.com
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download What Is The Electrons Are Shared Between Two Atoms The electrons involved are in the outer shells of the atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. An atom that shares one or more of its electrons will complete its outer. Usually, sharing electrons gives each atom a full valence shell and. According to valence bond. What Is The Electrons Are Shared Between Two Atoms.
From www.science-revision.co.uk
Covalent bonding What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Let us illustrate a covalent bond by using. An atom that shares one or more of its electrons will complete its outer. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. The. What Is The Electrons Are Shared Between Two Atoms.
From www.slideserve.com
PPT Identify that some electrons in solids are shared between atoms What Is The Electrons Are Shared Between Two Atoms The electrons involved are in the outer shells of the atoms. Usually, sharing electrons gives each atom a full valence shell and. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond.. What Is The Electrons Are Shared Between Two Atoms.
From sphweb.bumc.bu.edu
Basic Cell Biology What Is The Electrons Are Shared Between Two Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. It determines how the shared. An atom that shares one or more of its electrons will complete its outer. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. According to valence bond (vb) theory,. What Is The Electrons Are Shared Between Two Atoms.
From www.nagwa.com
Question Video Determining How Many Electrons Are Shared in a Single What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. It determines how the shared. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and. What Is The Electrons Are Shared Between Two Atoms.
From masterconceptsinchemistry.com
How hydrogen atoms share valence electrons to form covalent bond and What Is The Electrons Are Shared Between Two Atoms The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. It determines how the shared. When electrons are shared between two atoms, they make a bond called a covalent bond. An atom that shares one or more of its electrons will complete its outer. According to valence bond (vb) theory,. What Is The Electrons Are Shared Between Two Atoms.
From mammothmemory.net
Covalent bonding is where atoms share electrons by bonding What Is The Electrons Are Shared Between Two Atoms The electrons involved are in the outer shells of the atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. When electrons are shared between two atoms, they make a bond called a covalent bond. It determines how the shared. An atom that shares one or more of its. What Is The Electrons Are Shared Between Two Atoms.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. The electrons involved are in the outer shells of the atoms. It. What Is The Electrons Are Shared Between Two Atoms.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Is The Electrons Are Shared Between Two Atoms The electrons involved are in the outer shells of the atoms. It determines how the shared. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. An atom that shares one or more of its electrons will complete its outer. Let. What Is The Electrons Are Shared Between Two Atoms.
From masterconceptsinchemistry.com
How hydrogen atoms share valence electrons to form covalent bond and What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. The electrons involved are in the outer shells of the atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a full valence shell and. An atom that shares. What Is The Electrons Are Shared Between Two Atoms.
From thescienceandmathszone.com
Covalent Bonding The Science and Maths Zone What Is The Electrons Are Shared Between Two Atoms It determines how the shared. The electrons involved are in the outer shells of the atoms. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron. What Is The Electrons Are Shared Between Two Atoms.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram What Is The Electrons Are Shared Between Two Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using. The electrons involved are in the outer shells of the atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. It determines how the shared. A. What Is The Electrons Are Shared Between Two Atoms.
From www.expii.com
Multiple Bonds — Double & Triple Bonds Expii What Is The Electrons Are Shared Between Two Atoms According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. Let us illustrate a covalent bond by using. An atom that shares one or more of its electrons will complete its outer. When electrons are shared between two atoms, they make. What Is The Electrons Are Shared Between Two Atoms.
From www.expii.com
Electrons — Structure & Properties Expii What Is The Electrons Are Shared Between Two Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a full valence shell and. Let us illustrate a covalent bond by using. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. The electrons involved are in the. What Is The Electrons Are Shared Between Two Atoms.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is The Electrons Are Shared Between Two Atoms When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a full valence shell and. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. The sharing of a pair of electrons represents a single covalent bond, usually just referred to. What Is The Electrons Are Shared Between Two Atoms.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. When electrons are shared between two atoms, they make a bond called a covalent bond. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps. What Is The Electrons Are Shared Between Two Atoms.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent What Is The Electrons Are Shared Between Two Atoms The electrons involved are in the outer shells of the atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. A covalent bond is a chemical bond between. What Is The Electrons Are Shared Between Two Atoms.
From www.slideserve.com
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation ID5979513 What Is The Electrons Are Shared Between Two Atoms The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. The electrons involved are in the outer shells of the atoms. Usually, sharing electrons gives each atom a full valence shell and. It determines how the shared. When electrons are shared between two atoms, they make a bond called a. What Is The Electrons Are Shared Between Two Atoms.
From www.broadlearnings.com
Atomic Structure Broad Learnings What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. When electrons are shared between two atoms, they make a bond called a covalent bond. It determines how the shared. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Let. What Is The Electrons Are Shared Between Two Atoms.
From www.pinterest.com
What Happens When Atoms Bond infographic diagram showing how electrons What Is The Electrons Are Shared Between Two Atoms Let us illustrate a covalent bond by using. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. When electrons are shared between two atoms, they make a bond called a covalent bond. The electrons involved are in the outer shells of the atoms. An atom that shares one or more. What Is The Electrons Are Shared Between Two Atoms.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Is The Electrons Are Shared Between Two Atoms It determines how the shared. When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a full valence shell and. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. A covalent bond is a chemical bond between two atoms where. What Is The Electrons Are Shared Between Two Atoms.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.44 Know that a Covalent Bond is Formed Between What Is The Electrons Are Shared Between Two Atoms An atom that shares one or more of its electrons will complete its outer. Usually, sharing electrons gives each atom a full valence shell and. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. It determines how the shared. When electrons are shared between two atoms, they make a bond. What Is The Electrons Are Shared Between Two Atoms.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts What Is The Electrons Are Shared Between Two Atoms Usually, sharing electrons gives each atom a full valence shell and. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Let us illustrate a covalent bond by using. It determines how the shared. The electrons involved are in the outer shells of the atoms. An atom that shares one. What Is The Electrons Are Shared Between Two Atoms.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is The Electrons Are Shared Between Two Atoms According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. It determines how the shared. Usually, sharing electrons gives each atom a full valence shell and. The sharing of a pair of electrons represents a single covalent bond, usually just referred. What Is The Electrons Are Shared Between Two Atoms.
From www2.chemistry.msu.edu
Electron Configurations & The Periodic Table What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. An atom that shares one or more of its electrons will complete. What Is The Electrons Are Shared Between Two Atoms.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID What Is The Electrons Are Shared Between Two Atoms The electrons involved are in the outer shells of the atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a full valence shell and. Let us illustrate a covalent bond by using. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron. What Is The Electrons Are Shared Between Two Atoms.
From www.slideserve.com
PPT Covalent bonds where electrons are shared PowerPoint What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. When electrons are shared between two atoms, they make a bond called a covalent bond. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps. What Is The Electrons Are Shared Between Two Atoms.
From www.numerade.com
SOLVED Text shared between two atoms? 17. In which of the structures What Is The Electrons Are Shared Between Two Atoms The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. The electrons involved are in the outer shells of the atoms. An atom that shares one or more of its electrons will. What Is The Electrons Are Shared Between Two Atoms.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology What Is The Electrons Are Shared Between Two Atoms A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. When electrons are shared between two atoms, they make a bond called. What Is The Electrons Are Shared Between Two Atoms.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation What Is The Electrons Are Shared Between Two Atoms It determines how the shared. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. Usually, sharing electrons gives each atom a full valence shell and. According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps. What Is The Electrons Are Shared Between Two Atoms.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together What Is The Electrons Are Shared Between Two Atoms The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. When electrons are shared between two atoms, they make a bond called a covalent bond. Usually, sharing electrons gives each atom a. What Is The Electrons Are Shared Between Two Atoms.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Is The Electrons Are Shared Between Two Atoms Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. An atom that shares one or more of its electrons will complete its outer. When electrons are shared between two atoms, they make a bond called a covalent bond. The electrons involved are in the outer shells of the atoms. Let us. What Is The Electrons Are Shared Between Two Atoms.
From www.numerade.com
⏩SOLVEDHow many electrons are shared between two atoms in (a) a What Is The Electrons Are Shared Between Two Atoms According to valence bond (vb) theory, a covalent bond forms when two atoms approach each other closely and a singly occupied orbital on one atom overlaps a singly occupied. The electrons involved are in the outer shells of the atoms. A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually,. What Is The Electrons Are Shared Between Two Atoms.