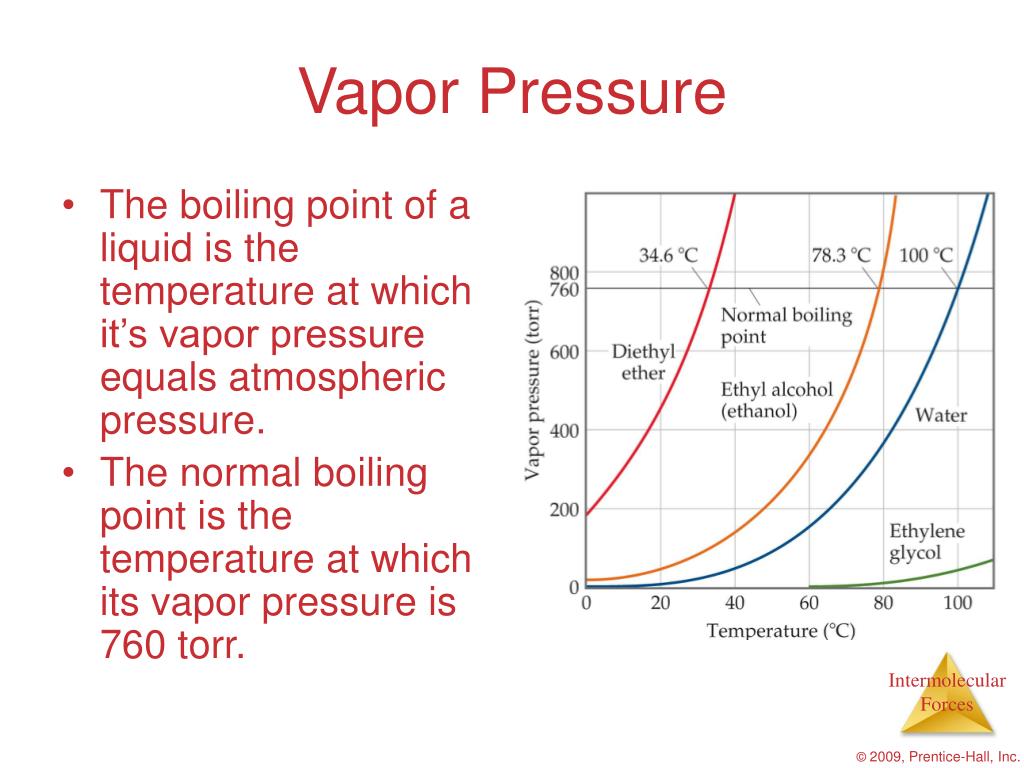
Evaporation Increase Pressure . The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Strong intermolecular forces produce a lower rate of evaporation and a. There are two factors that influence the vapor pressure: Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.
from www.slideserve.com
As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. There are two factors that influence the vapor pressure: Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and a.
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247
Evaporation Increase Pressure If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Strong intermolecular forces produce a lower rate of evaporation and a. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. There are two factors that influence the vapor pressure: If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance.
From socratic.org
How does atmospheric pressure change with altitude? Socratic Evaporation Increase Pressure If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container.. Evaporation Increase Pressure.
From www.researchgate.net
A. Relationship between vapor pressure, relative humidity and... Download Scientific Diagram Evaporation Increase Pressure Strong intermolecular forces produce a lower rate of evaporation and a. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance.. Evaporation Increase Pressure.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube Evaporation Increase Pressure Strong intermolecular forces produce a lower rate of evaporation and a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in. Evaporation Increase Pressure.
From saylordotorg.github.io
Vapor Pressure Evaporation Increase Pressure The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. There are two factors that influence the vapor pressure: The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation. Evaporation Increase Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Evaporation Increase Pressure There are two factors that influence the vapor pressure: The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation. Evaporation Increase Pressure.
From www.youtube.com
Vapor Pressure, Equilibrium Vapor Pressure, and Relative Humidity YouTube Evaporation Increase Pressure The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor. Evaporation Increase Pressure.
From www.researchgate.net
1 The atmospheric density and pressure distribution. Download Scientific Diagram Evaporation Increase Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. The higher the temperature is, the more molecules have enough energy to escape from the liquid. Evaporation Increase Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Evaporation Increase Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor. Evaporation Increase Pressure.
From thewaterfiltermarket.com
How Long Does It Take for Water To Evaporate? Water Filter Market Evaporation Increase Pressure If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. Strong intermolecular forces produce a lower rate of evaporation and a. There are two factors that influence the vapor pressure: The higher the temperature is, the more molecules have enough energy to escape from. Evaporation Increase Pressure.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel Evaporation Increase Pressure Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the. Evaporation Increase Pressure.
From www.slideshare.net
Chapter 11 Properties of Solutions Evaporation Increase Pressure Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. There are two factors that influence the vapor pressure: The higher the temperature is, the more molecules have. Evaporation Increase Pressure.
From www.youtube.com
Vapor Pressure and Boiling YouTube Evaporation Increase Pressure The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. There are two factors that influence the vapor pressure: Strong intermolecular forces produce a lower rate of evaporation and a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the. Evaporation Increase Pressure.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Increase Pressure If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it. Evaporation Increase Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Evaporation Increase Pressure The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. If a lid is placed over the container, as in figure(b), evaporation continues, increasing. Evaporation Increase Pressure.
From jmpcoblog.com
Avoiding Pump Cavitation in Open Systems The Dynamic Relationship Between NPSH, Vapor Pressure Evaporation Increase Pressure Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure. Evaporation Increase Pressure.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Evaporation Increase Pressure Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient. Evaporation Increase Pressure.
From mavink.com
Vapor Pressure And Boiling Point Relationship Evaporation Increase Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. There are two factors that influence the vapor pressure: The higher. Evaporation Increase Pressure.
From serc.carleton.edu
Phase Rule Evaporation Increase Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Strong intermolecular forces produce a lower rate of evaporation and a. Vapor pressure is a measure of the pressure exerted by a. Evaporation Increase Pressure.
From www.chemistrylearner.com
Saturated Vapor Pressure Definition and Temperature Effect Evaporation Increase Pressure Strong intermolecular forces produce a lower rate of evaporation and a. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. There are two factors that influence the vapor. Evaporation Increase Pressure.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor... Download Scientific Evaporation Increase Pressure Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Strong intermolecular forces produce a lower rate of evaporation and a. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. As the temperature of a liquid increases, the vapor pressure of the liquid increases until. Evaporation Increase Pressure.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Evaporation Increase Pressure Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. The. Evaporation Increase Pressure.
From www.researchgate.net
For two different evaporation temperatures the dependence of the... Download Scientific Diagram Evaporation Increase Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation. Evaporation Increase Pressure.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Increase Pressure Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. Strong intermolecular forces produce a lower rate of evaporation and a. There are two factors that influence the vapor pressure: The vapor pressure. Evaporation Increase Pressure.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Evaporation Increase Pressure There are two factors that influence the vapor pressure: As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Strong intermolecular forces produce a lower rate of evaporation and a. The vapor. Evaporation Increase Pressure.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Evaporation Increase Pressure If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it. Evaporation Increase Pressure.
From www.researchgate.net
Evolution of water vapor pressure Pvapor along with interfacial... Download Scientific Diagram Evaporation Increase Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid);. Strong intermolecular forces produce a lower rate of evaporation and a. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. As the temperature of a. Evaporation Increase Pressure.
From www.slideserve.com
PPT 17 Equilibrium (AHL) PowerPoint Presentation, free download ID2464656 Evaporation Increase Pressure Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. Strong intermolecular forces produce a lower rate of evaporation and a. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it. Evaporation Increase Pressure.
From vacaero.com
Evaporation Evaporation Increase Pressure If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and a. As the temperature of. Evaporation Increase Pressure.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 Evaporation Increase Pressure Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. Strong intermolecular forces produce a lower rate of evaporation and a. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Generally a substance's vapor pressure increases as. Evaporation Increase Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Evaporation Increase Pressure Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. As the temperature of a liquid increases, the vapor pressure of the liquid increases until. Evaporation Increase Pressure.
From www.slideserve.com
PPT Unit 3 Phase Changes & Energy PowerPoint Presentation, free download ID3721071 Evaporation Increase Pressure If a lid is placed over the container, as in figure(b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance. Strong intermolecular forces produce a lower rate of evaporation and a. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate.. Evaporation Increase Pressure.
From socratic.org
What do we mean by a dynamic equilibrium? Can you describe how the development of a vapor Evaporation Increase Pressure The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. The higher the temperature is, the more molecules have enough energy to escape from the liquid or solid,. There are two factors that influence the vapor pressure: The vapor pressure of a liquid is the equilibrium pressure. Evaporation Increase Pressure.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Evaporation Increase Pressure Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation. Evaporation Increase Pressure.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free download ID1623857 Evaporation Increase Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Vapor pressure is a measure of the pressure exerted by a gas above a liquid in a sealed container. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation. Evaporation Increase Pressure.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download ID6813105 Evaporation Increase Pressure Strong intermolecular forces produce a lower rate of evaporation and a. The vapor pressure stays the same as the surface area increases because the rates of evaporation and condensation increase at the same rate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of a liquid increases, the vapor pressure of. Evaporation Increase Pressure.