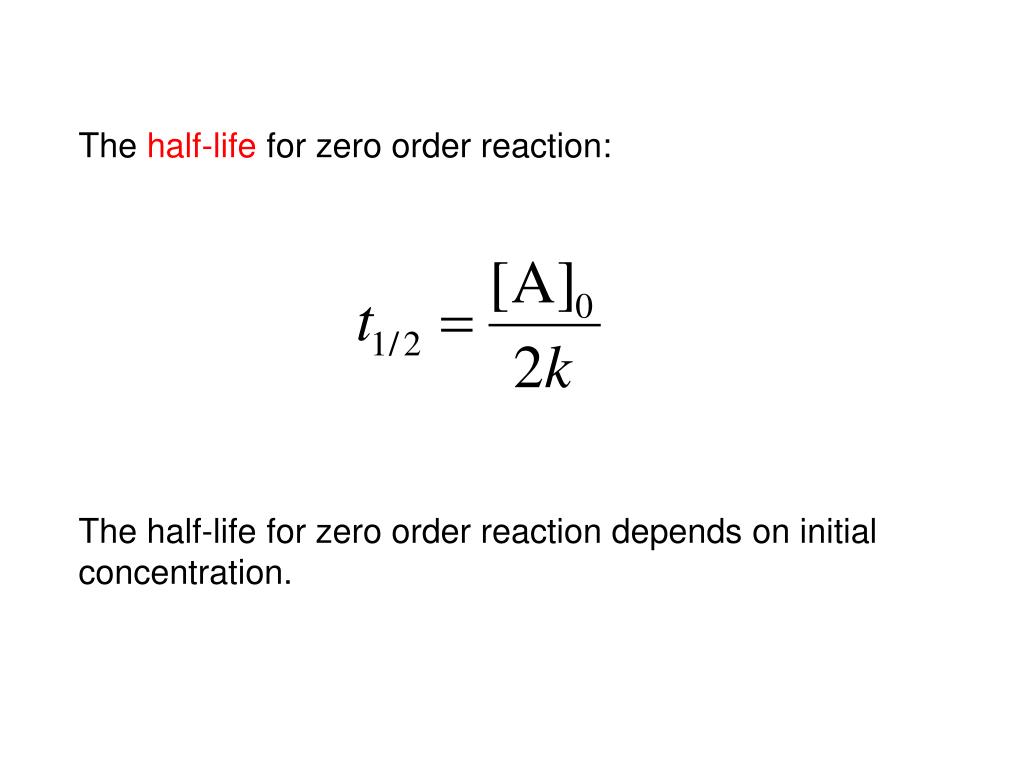
Half Life Equation For Third Order Reaction . For a zero order reaction a products , rate = k: Converting a half life to a rate constant. T ½ = [a o] / 2k. For a first order reaction a products , rate =. Identify the order of a reaction from concentration/time. Da dt = −ka3 d a d t = − k a 3. T = 1/ ( 2ka2), which is evaluated at the two times in question. This integrates up to give: Graphical relations and half lives.
from www.slideserve.com
Identify the order of a reaction from concentration/time. Da dt = −ka3 d a d t = − k a 3. For a first order reaction a products , rate =. T ½ = [a o] / 2k. Converting a half life to a rate constant. For a zero order reaction a products , rate = k: Graphical relations and half lives. This integrates up to give: T = 1/ ( 2ka2), which is evaluated at the two times in question.
PPT Chapter 13 Chemical PowerPoint Presentation, free
Half Life Equation For Third Order Reaction This integrates up to give: Graphical relations and half lives. T = 1/ ( 2ka2), which is evaluated at the two times in question. For a zero order reaction a products , rate = k: This integrates up to give: Da dt = −ka3 d a d t = − k a 3. Converting a half life to a rate constant. Identify the order of a reaction from concentration/time. For a first order reaction a products , rate =. T ½ = [a o] / 2k.
From www.chegg.com
Solved a) Derive the half life equation for a third order Half Life Equation For Third Order Reaction T = 1/ ( 2ka2), which is evaluated at the two times in question. Identify the order of a reaction from concentration/time. Converting a half life to a rate constant. For a zero order reaction a products , rate = k: For a first order reaction a products , rate =. Graphical relations and half lives. This integrates up to. Half Life Equation For Third Order Reaction.
From www.youtube.com
How to Calculate HalfLife for Second Order Reactions YouTube Half Life Equation For Third Order Reaction T ½ = [a o] / 2k. T = 1/ ( 2ka2), which is evaluated at the two times in question. For a zero order reaction a products , rate = k: Converting a half life to a rate constant. Graphical relations and half lives. Identify the order of a reaction from concentration/time. This integrates up to give: Da dt. Half Life Equation For Third Order Reaction.
From lessonmagicpassaged.z21.web.core.windows.net
Calculating Half Life Worksheet Half Life Equation For Third Order Reaction T ½ = [a o] / 2k. T = 1/ ( 2ka2), which is evaluated at the two times in question. Identify the order of a reaction from concentration/time. For a first order reaction a products , rate =. Converting a half life to a rate constant. Graphical relations and half lives. Da dt = −ka3 d a d t. Half Life Equation For Third Order Reaction.
From slideplayer.com
Chemical Introduction ppt download Half Life Equation For Third Order Reaction Converting a half life to a rate constant. Da dt = −ka3 d a d t = − k a 3. For a zero order reaction a products , rate = k: Graphical relations and half lives. T ½ = [a o] / 2k. Identify the order of a reaction from concentration/time. For a first order reaction a products ,. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Chemical Chapter 14 PowerPoint Presentation, free Half Life Equation For Third Order Reaction Graphical relations and half lives. For a first order reaction a products , rate =. Converting a half life to a rate constant. This integrates up to give: T ½ = [a o] / 2k. T = 1/ ( 2ka2), which is evaluated at the two times in question. For a zero order reaction a products , rate = k:. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Half Life Equation For Third Order Reaction For a first order reaction a products , rate =. Da dt = −ka3 d a d t = − k a 3. T = 1/ ( 2ka2), which is evaluated at the two times in question. Graphical relations and half lives. This integrates up to give: Converting a half life to a rate constant. For a zero order reaction. Half Life Equation For Third Order Reaction.
From www.studocu.com
Lesson 20 Halflife equations for 1st 2nd and 3rd order. 1 Lesson 20 Half Life Equation For Third Order Reaction Converting a half life to a rate constant. T = 1/ ( 2ka2), which is evaluated at the two times in question. Identify the order of a reaction from concentration/time. For a zero order reaction a products , rate = k: For a first order reaction a products , rate =. T ½ = [a o] / 2k. Graphical relations. Half Life Equation For Third Order Reaction.
From www.slideshare.net
General chemistry ii chapter 14 Half Life Equation For Third Order Reaction T = 1/ ( 2ka2), which is evaluated at the two times in question. T ½ = [a o] / 2k. Converting a half life to a rate constant. For a first order reaction a products , rate =. Identify the order of a reaction from concentration/time. Graphical relations and half lives. This integrates up to give: Da dt =. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Chemical Chapter 14 PowerPoint Presentation, free Half Life Equation For Third Order Reaction T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: Da dt = −ka3 d a d t = − k a 3. Identify the order of a reaction from concentration/time. Graphical relations and half lives. T = 1/ ( 2ka2), which is evaluated at the two times in question. For a. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Half Life Equation For Third Order Reaction Graphical relations and half lives. This integrates up to give: T = 1/ ( 2ka2), which is evaluated at the two times in question. Da dt = −ka3 d a d t = − k a 3. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: Converting a half life to. Half Life Equation For Third Order Reaction.
From www.youtube.com
The Half Life YouTube Half Life Equation For Third Order Reaction Identify the order of a reaction from concentration/time. For a first order reaction a products , rate =. For a zero order reaction a products , rate = k: Da dt = −ka3 d a d t = − k a 3. T ½ = [a o] / 2k. This integrates up to give: T = 1/ ( 2ka2), which. Half Life Equation For Third Order Reaction.
From general.chemistrysteps.com
HalfLife of a Reaction Chemistry Steps Half Life Equation For Third Order Reaction Identify the order of a reaction from concentration/time. Graphical relations and half lives. For a first order reaction a products , rate =. Converting a half life to a rate constant. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: This integrates up to give: Da dt = −ka3 d a. Half Life Equation For Third Order Reaction.
From classnotes.org.in
Half Life Period of a Reaction Chemical Chemistry, Class 12 Half Life Equation For Third Order Reaction For a zero order reaction a products , rate = k: Converting a half life to a rate constant. Da dt = −ka3 d a d t = − k a 3. Identify the order of a reaction from concentration/time. Graphical relations and half lives. T = 1/ ( 2ka2), which is evaluated at the two times in question. For. Half Life Equation For Third Order Reaction.
From www.youtube.com
Integrated Rate Equation for third Order Reaction Third Order Half Life Equation For Third Order Reaction For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. T = 1/ ( 2ka2), which is evaluated at the two times in question. Identify the order of a reaction from concentration/time. For a first order reaction a products , rate =. This integrates up to give: Graphical relations and half lives.. Half Life Equation For Third Order Reaction.
From mapleschilling.blogspot.com
half life formula physics Maple Schilling Half Life Equation For Third Order Reaction Graphical relations and half lives. T ½ = [a o] / 2k. Identify the order of a reaction from concentration/time. For a zero order reaction a products , rate = k: For a first order reaction a products , rate =. Converting a half life to a rate constant. T = 1/ ( 2ka2), which is evaluated at the two. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Geochemical PowerPoint Presentation, free download ID Half Life Equation For Third Order Reaction T ½ = [a o] / 2k. This integrates up to give: For a first order reaction a products , rate =. For a zero order reaction a products , rate = k: T = 1/ ( 2ka2), which is evaluated at the two times in question. Graphical relations and half lives. Converting a half life to a rate constant.. Half Life Equation For Third Order Reaction.
From www.numerade.com
SOLVED The rate equation for a 3rdorder reaction 3A >products is Half Life Equation For Third Order Reaction T ½ = [a o] / 2k. Graphical relations and half lives. Da dt = −ka3 d a d t = − k a 3. Identify the order of a reaction from concentration/time. T = 1/ ( 2ka2), which is evaluated at the two times in question. For a first order reaction a products , rate =. Converting a half. Half Life Equation For Third Order Reaction.
From www.youtube.com
integrated rate law and halflife expression for general Nth Half Life Equation For Third Order Reaction Da dt = −ka3 d a d t = − k a 3. Graphical relations and half lives. Identify the order of a reaction from concentration/time. T ½ = [a o] / 2k. Converting a half life to a rate constant. T = 1/ ( 2ka2), which is evaluated at the two times in question. This integrates up to give:. Half Life Equation For Third Order Reaction.
From www.youtube.com
Halflife of a firstorder reaction AP Chemistry Khan Half Life Equation For Third Order Reaction For a first order reaction a products , rate =. For a zero order reaction a products , rate = k: This integrates up to give: Identify the order of a reaction from concentration/time. Graphical relations and half lives. T ½ = [a o] / 2k. T = 1/ ( 2ka2), which is evaluated at the two times in question.. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Half Life Equation For Third Order Reaction For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. Identify the order of a reaction from concentration/time. Converting a half life to a rate constant. Graphical relations and half lives. T = 1/ ( 2ka2), which is evaluated at the two times in question. This integrates up to give: For a. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Enzyme and Catalysis PowerPoint Presentation, free Half Life Equation For Third Order Reaction Graphical relations and half lives. T = 1/ ( 2ka2), which is evaluated at the two times in question. For a first order reaction a products , rate =. For a zero order reaction a products , rate = k: Da dt = −ka3 d a d t = − k a 3. This integrates up to give: Converting a. Half Life Equation For Third Order Reaction.
From www.chegg.com
Solved 2. a) Derive the half life equation for a third order Half Life Equation For Third Order Reaction T ½ = [a o] / 2k. T = 1/ ( 2ka2), which is evaluated at the two times in question. Converting a half life to a rate constant. This integrates up to give: For a zero order reaction a products , rate = k: Da dt = −ka3 d a d t = − k a 3. Graphical relations. Half Life Equation For Third Order Reaction.
From www.youtube.com
Derive Expression for Half Life of 3rd order reaction Chemical Half Life Equation For Third Order Reaction For a first order reaction a products , rate =. Converting a half life to a rate constant. T = 1/ ( 2ka2), which is evaluated at the two times in question. T ½ = [a o] / 2k. This integrates up to give: Da dt = −ka3 d a d t = − k a 3. For a zero. Half Life Equation For Third Order Reaction.
From www.studypool.com
SOLUTION 2 derive the following half life equation for the nth order Half Life Equation For Third Order Reaction Da dt = −ka3 d a d t = − k a 3. For a zero order reaction a products , rate = k: Converting a half life to a rate constant. T = 1/ ( 2ka2), which is evaluated at the two times in question. Identify the order of a reaction from concentration/time. For a first order reaction a. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Chapter 14 Chemical PowerPoint Presentation, free Half Life Equation For Third Order Reaction Graphical relations and half lives. Identify the order of a reaction from concentration/time. For a zero order reaction a products , rate = k: Da dt = −ka3 d a d t = − k a 3. T = 1/ ( 2ka2), which is evaluated at the two times in question. This integrates up to give: T ½ = [a. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5829521 Half Life Equation For Third Order Reaction Identify the order of a reaction from concentration/time. T = 1/ ( 2ka2), which is evaluated at the two times in question. For a zero order reaction a products , rate = k: Graphical relations and half lives. This integrates up to give: Da dt = −ka3 d a d t = − k a 3. For a first order. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID1306821 Half Life Equation For Third Order Reaction T = 1/ ( 2ka2), which is evaluated at the two times in question. Graphical relations and half lives. This integrates up to give: Identify the order of a reaction from concentration/time. Da dt = −ka3 d a d t = − k a 3. For a zero order reaction a products , rate = k: T ½ = [a. Half Life Equation For Third Order Reaction.
From chemistry.stackexchange.com
organic chemistry Halflife and shelflife of secondorder reaction Half Life Equation For Third Order Reaction T = 1/ ( 2ka2), which is evaluated at the two times in question. Identify the order of a reaction from concentration/time. T ½ = [a o] / 2k. Graphical relations and half lives. For a first order reaction a products , rate =. For a zero order reaction a products , rate = k: Converting a half life to. Half Life Equation For Third Order Reaction.
From www.chegg.com
Solved = 2 Halflife of thirdorder Reaction t2 Half Life Equation For Third Order Reaction Converting a half life to a rate constant. For a zero order reaction a products , rate = k: This integrates up to give: Identify the order of a reaction from concentration/time. Graphical relations and half lives. Da dt = −ka3 d a d t = − k a 3. T ½ = [a o] / 2k. For a first. Half Life Equation For Third Order Reaction.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Half Life Equation For Third Order Reaction Da dt = −ka3 d a d t = − k a 3. For a first order reaction a products , rate =. Converting a half life to a rate constant. Graphical relations and half lives. T = 1/ ( 2ka2), which is evaluated at the two times in question. This integrates up to give: T ½ = [a o]. Half Life Equation For Third Order Reaction.
From slideplayer.com
Chapter 15 Chemical ppt download Half Life Equation For Third Order Reaction For a first order reaction a products , rate =. This integrates up to give: Da dt = −ka3 d a d t = − k a 3. Graphical relations and half lives. Identify the order of a reaction from concentration/time. For a zero order reaction a products , rate = k: T = 1/ ( 2ka2), which is evaluated. Half Life Equation For Third Order Reaction.
From byjus.com
Half life of a certain zero order reaction, A → P is 2 hour when the Half Life Equation For Third Order Reaction Da dt = −ka3 d a d t = − k a 3. This integrates up to give: Graphical relations and half lives. For a first order reaction a products , rate =. Identify the order of a reaction from concentration/time. T ½ = [a o] / 2k. T = 1/ ( 2ka2), which is evaluated at the two times. Half Life Equation For Third Order Reaction.
From www.youtube.com
Half Life Equation Derivation YouTube Half Life Equation For Third Order Reaction T = 1/ ( 2ka2), which is evaluated at the two times in question. T ½ = [a o] / 2k. Identify the order of a reaction from concentration/time. This integrates up to give: For a first order reaction a products , rate =. Graphical relations and half lives. Converting a half life to a rate constant. For a zero. Half Life Equation For Third Order Reaction.
From www.reddit.com
How to derive halflife equation r/chemistryhelp Half Life Equation For Third Order Reaction Identify the order of a reaction from concentration/time. T ½ = [a o] / 2k. Converting a half life to a rate constant. Graphical relations and half lives. This integrates up to give: Da dt = −ka3 d a d t = − k a 3. For a first order reaction a products , rate =. T = 1/ (. Half Life Equation For Third Order Reaction.
From ar.inspiredpencil.com
Half Life Formula Chemistry Half Life Equation For Third Order Reaction Identify the order of a reaction from concentration/time. For a first order reaction a products , rate =. For a zero order reaction a products , rate = k: Da dt = −ka3 d a d t = − k a 3. Converting a half life to a rate constant. T = 1/ ( 2ka2), which is evaluated at the. Half Life Equation For Third Order Reaction.