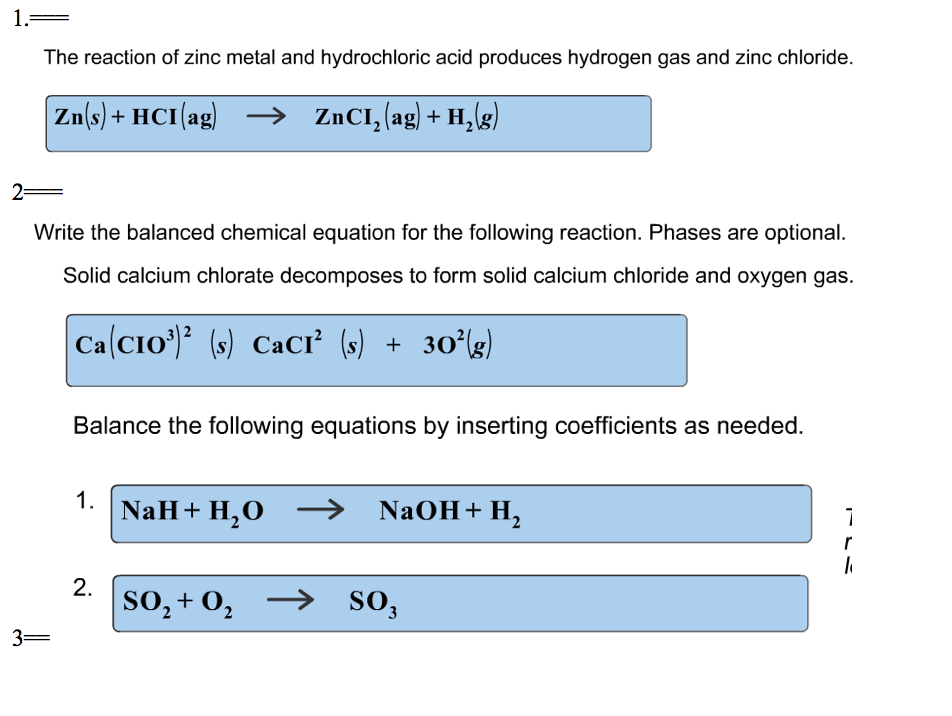
Difference Between Zinc And Hydrochloric Acid . Zinc reacts with halogens in the presence of moisture: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. Zn + cl₂ → zncl₂. Some other reactions absorb energy. The reaction between zinc metal and the. Zn(s) + 2hcl(aq) → zncl2(aq). For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Hydrochloric acid + zinc → zinc chloride +. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal.
from slidesharetrick.blogspot.com
When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Acid + metal → salt + hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq). Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Some other reactions absorb energy. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The reaction between zinc metal and the. Zn + cl₂ → zncl₂. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zinc reacts with halogens in the presence of moisture:
Zinc And Hydrochloric Acid slidesharetrick
Difference Between Zinc And Hydrochloric Acid Acid + metal → salt + hydrogen. Acid + metal → salt + hydrogen. This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Zn + cl₂ → zncl₂. Zn(s) + 2hcl(aq) → zncl2(aq). Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zinc reacts with halogens in the presence of moisture: The reaction between zinc metal and the. Some other reactions absorb energy. Hydrochloric acid + zinc → zinc chloride +.
From warreninstitute.org
Unlock The POWER Of Chemical Reaction Zn + HCl To Zinc + Hydrochloric Acid Difference Between Zinc And Hydrochloric Acid Some other reactions absorb energy. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Zn(s) + 2hcl(aq) → zncl2(aq). Zn + cl₂ → zncl₂. Hydrochloric acid + zinc → zinc chloride +. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Zinc reacts steadily with. Difference Between Zinc And Hydrochloric Acid.
From spark.adobe.com
Zinc and Hydrochloric Acid Difference Between Zinc And Hydrochloric Acid This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Zn(s) + 2hcl(aq) → zncl2(aq). Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Hydrochloric acid + zinc → zinc chloride +. Zn +. Difference Between Zinc And Hydrochloric Acid.
From slideplayer.com
Chemical Reactions & Equations ppt download Difference Between Zinc And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Some other reactions absorb energy. Zn + cl₂ → zncl₂. The reaction between zinc metal and the. Acid + metal → salt + hydrogen. Zinc reacts with. Difference Between Zinc And Hydrochloric Acid.
From spark.adobe.com
Zinc and Hydrochloric Acid Difference Between Zinc And Hydrochloric Acid This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Some other reactions absorb energy. The reaction between zinc metal and the. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate. Difference Between Zinc And Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Science Photo Library Difference Between Zinc And Hydrochloric Acid This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. Zinc reacts with halogens in the presence of moisture: When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Acid + metal → salt + hydrogen. For example, zinc metal reacts with hydrochloric. Difference Between Zinc And Hydrochloric Acid.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Difference Between Zinc And Hydrochloric Acid Zn + cl₂ → zncl₂. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Zinc reacts with halogens in the presence of moisture: This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Acids will react with reactive metals, such as magnesium and zinc, to make. Difference Between Zinc And Hydrochloric Acid.
From spark.adobe.com
Zinc and Hydrochloric Acid Difference Between Zinc And Hydrochloric Acid When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Some other reactions absorb energy. The reaction between zinc metal and the. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Zn + cl₂ → zncl₂. Zn(s) + 2hcl(aq) → zncl2(aq). Acids will react with reactive. Difference Between Zinc And Hydrochloric Acid.
From melscience.com
The reaction between hydrochloric acid and zinc MEL Chemistry Difference Between Zinc And Hydrochloric Acid Zinc reacts with halogens in the presence of moisture: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Some other reactions absorb energy. Zn(s) + 2hcl(aq) → zncl2(aq). Acid + metal → salt + hydrogen. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. This chemistry video. Difference Between Zinc And Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0622 Science Photo Library Difference Between Zinc And Hydrochloric Acid Some other reactions absorb energy. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq). Zn + cl₂ → zncl₂. When zinc reacts with hydrochloric acid, the test tube. Difference Between Zinc And Hydrochloric Acid.
From www.youtube.com
Chemical Reaction zinc and hydrochloric acid YouTube Difference Between Zinc And Hydrochloric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq). When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction.. Difference Between Zinc And Hydrochloric Acid.
From www.coursehero.com
[Solved] Consider the reaction of zinc metal with hydrochloric acid Zn +... Course Hero Difference Between Zinc And Hydrochloric Acid Some other reactions absorb energy. Hydrochloric acid + zinc → zinc chloride +. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The reaction between zinc metal and the. For example, zinc metal. Difference Between Zinc And Hydrochloric Acid.
From jaylongokelawrence.blogspot.com
Zinc Reacts With Hydrochloric Acid Difference Between Zinc And Hydrochloric Acid The reaction between zinc metal and the. Zn(s) + 2hcl(aq) → zncl2(aq). Acid + metal → salt + hydrogen. Zn + cl₂ → zncl₂. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Some other reactions absorb energy. Zinc reacts with halogens in the presence of moisture: When zinc reacts with hydrochloric acid, the test. Difference Between Zinc And Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Photographs The Art of Difference Between Zinc And Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride +. This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. Zinc reacts with halogens in the presence of moisture: Zn(s) + 2hcl(aq) → zncl2(aq). This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. For example, zinc metal reacts with. Difference Between Zinc And Hydrochloric Acid.
From slidesharetrick.blogspot.com
Zinc And Hydrochloric Acid slidesharetrick Difference Between Zinc And Hydrochloric Acid Zn + cl₂ → zncl₂. Some other reactions absorb energy. Zinc reacts with halogens in the presence of moisture: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Hydrochloric acid + zinc → zinc chloride +. This chemistry video tutorial explains how to predict the products of the single replacement reaction between. Difference Between Zinc And Hydrochloric Acid.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Difference Between Zinc And Hydrochloric Acid The reaction between zinc metal and the. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Zn + cl₂ → zncl₂. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Hydrochloric acid + zinc → zinc chloride +. Zn(s) + 2hcl(aq) → zncl2(aq). This chemistry. Difference Between Zinc And Hydrochloric Acid.
From www.numerade.com
SOLVED Consider the reaction between zinc metal and hydrochloric acid. Which of the following Difference Between Zinc And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride. Difference Between Zinc And Hydrochloric Acid.
From slidesharetrick.blogspot.com
Zinc And Hydrochloric Acid slidesharetrick Difference Between Zinc And Hydrochloric Acid This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. The reaction between zinc metal and the. Zn(s) + 2hcl(aq) → zncl2(aq). Zinc reacts with halogens in the presence of moisture: When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. This experiment. Difference Between Zinc And Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Photographs The Art of Difference Between Zinc And Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride +. This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Some other reactions absorb energy. Acid + metal → salt + hydrogen. For example, zinc metal reacts. Difference Between Zinc And Hydrochloric Acid.
From thewonderofscience.com
Reaction of zinc and hydrochloric acid (NY) — The Wonder of Science Difference Between Zinc And Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride +. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. Some other reactions absorb energy. Zinc reacts with halogens in the presence of moisture: This experiment aims. Difference Between Zinc And Hydrochloric Acid.
From slidesharetrick.blogspot.com
Zinc And Hydrochloric Acid slidesharetrick Difference Between Zinc And Hydrochloric Acid Some other reactions absorb energy. Zn + cl₂ → zncl₂. Hydrochloric acid + zinc → zinc chloride +. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. Zinc reacts with halogens in the presence of moisture: This. Difference Between Zinc And Hydrochloric Acid.
From www.youtube.com
Xth Class, Physical Science, Acids, Bases and SaltsReaction of Zinc with Hydrochloric Acid Difference Between Zinc And Hydrochloric Acid This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. Hydrochloric acid + zinc → zinc chloride +. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. The reaction between. Difference Between Zinc And Hydrochloric Acid.
From www.youtube.com
Reaction of Zinc with dilute hydrochloric acid or sulphuric acid....Zn+H2 SO4 /HCl ? YouTube Difference Between Zinc And Hydrochloric Acid When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Acid + metal → salt + hydrogen. Some other. Difference Between Zinc And Hydrochloric Acid.
From www.youtube.com
Zinc and Hydrochloric Acid Reaction chemistry grade10science chemicalreaction YouTube Difference Between Zinc And Hydrochloric Acid Zinc reacts with halogens in the presence of moisture: Acid + metal → salt + hydrogen. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Some other reactions absorb energy. The reaction between zinc metal and the. This chemistry video tutorial. Difference Between Zinc And Hydrochloric Acid.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Difference Between Zinc And Hydrochloric Acid Some other reactions absorb energy. The reaction between zinc metal and the. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and. Difference Between Zinc And Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Photographs The Art of Difference Between Zinc And Hydrochloric Acid When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Zn(s) + 2hcl(aq) → zncl2(aq). Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Zn + cl₂ → zncl₂. The reaction between zinc metal and the. This chemistry video tutorial explains. Difference Between Zinc And Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Photographs The Art of Difference Between Zinc And Hydrochloric Acid Some other reactions absorb energy. This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. The reaction between zinc. Difference Between Zinc And Hydrochloric Acid.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Difference Between Zinc And Hydrochloric Acid Zn + cl₂ → zncl₂. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. The reaction between zinc metal and the. Zinc reacts with halogens in the presence of moisture: Some other reactions absorb energy. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Zinc. Difference Between Zinc And Hydrochloric Acid.
From www.youtube.com
How to balance zinc and hydrochloric acid? YouTube Difference Between Zinc And Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride +. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zn + cl₂ → zncl₂. Some other reactions absorb energy. Acids will react with reactive metals, such as magnesium and zinc, to make a. Difference Between Zinc And Hydrochloric Acid.
From express.adobe.com
Zinc and Hydrochloric Acid Difference Between Zinc And Hydrochloric Acid Zn + cl₂ → zncl₂. Zn(s) + 2hcl(aq) → zncl2(aq). For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Some other reactions absorb energy. This chemistry video tutorial explains how to predict the products of the. Difference Between Zinc And Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Photo Library Difference Between Zinc And Hydrochloric Acid Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Some other reactions absorb energy. Zn + cl₂ → zncl₂. Hydrochloric acid + zinc → zinc chloride +. When zinc reacts with hydrochloric acid, the test tube. Difference Between Zinc And Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Photographs The Art of Difference Between Zinc And Hydrochloric Acid Zn(s) + 2hcl(aq) → zncl2(aq). Some other reactions absorb energy. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. This chemistry video tutorial explains how to predict the products of the single replacement. Difference Between Zinc And Hydrochloric Acid.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction between Zinc Metal and Difference Between Zinc And Hydrochloric Acid This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. When zinc reacts with hydrochloric acid, the test tube becomes very warm as energy is released during the reaction. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. For example, zinc metal reacts with. Difference Between Zinc And Hydrochloric Acid.
From www.youtube.com
Zinc and Hydrochloric Acid YouTube Difference Between Zinc And Hydrochloric Acid The reaction between zinc metal and the. Zn + cl₂ → zncl₂. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq). Acids will react with reactive metals, such as magnesium and. Difference Between Zinc And Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science Photo Library Difference Between Zinc And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. This chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc and. Zn(s) + 2hcl(aq) → zncl2(aq). Zinc reacts with halogens in the presence of moisture: Hydrochloric acid + zinc → zinc chloride +. Zn + cl₂ → zncl₂. When. Difference Between Zinc And Hydrochloric Acid.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq.) ZnCl2(aq.)+ H2(9) If 0. Difference Between Zinc And Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. This experiment aims to study the displacement reaction between hydrochloric acid with zinc metal. Some other reactions absorb energy. Zn(s) + 2hcl(aq) → zncl2(aq). This chemistry video tutorial explains. Difference Between Zinc And Hydrochloric Acid.