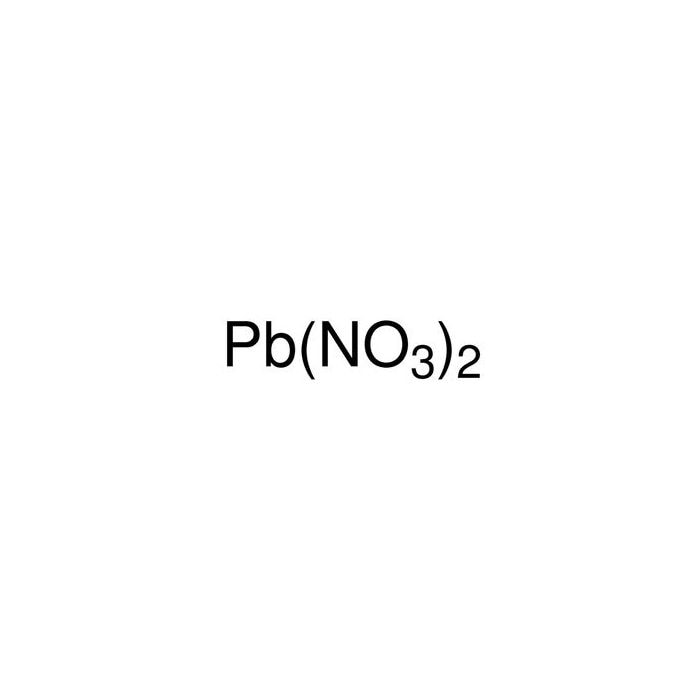
Lead (Ii) Nitrate And Lead Nitrate . It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. N 2 o 6 pb. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. Lead nitrate is also known as lead(ii) nitrate. Lead(ii) nitrate is toxic and must be handled with care. It is a chemical compound with the formula pb(no₃)₂. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Because of the insolubility of. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It is an oxidizing agent and is used to make other lead. This compound is highly soluble in water
from lab.honeywell.com
Lead nitrate is also known as lead(ii) nitrate. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. This compound is highly soluble in water Because of the insolubility of. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It is an oxidizing agent and is used to make other lead. It is a chemical compound with the formula pb(no₃)₂.
Lead(II) nitrate 228621 Honeywell Research Chemicals
Lead (Ii) Nitrate And Lead Nitrate Lead nitrate is also known as lead(ii) nitrate. This compound is highly soluble in water Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Lead(ii) nitrate is toxic and must be handled with care. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. Because of the insolubility of. Lead nitrate is also known as lead(ii) nitrate. It is an oxidizing agent and is used to make other lead. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It is a chemical compound with the formula pb(no₃)₂. N 2 o 6 pb.
From testbook.com
Lead (II) Nitrate Formula Structure, Preparation, & Uses Lead (Ii) Nitrate And Lead Nitrate It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. It is a chemical compound with the formula pb(no₃)₂. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Lead(ii) nitrate is toxic and must. Lead (Ii) Nitrate And Lead Nitrate.
From www.myxxgirl.com
Double Replacement Reaction Of Lead Ii Nitrate With Potassium Iodide Lead (Ii) Nitrate And Lead Nitrate N 2 o 6 pb. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It is an oxidizing agent and is used to make other lead. This compound is highly soluble in water It is a chemical compound with the formula pb(no₃)₂. Lead(ii) nitrate is toxic and must be handled with care. It describes. Lead (Ii) Nitrate And Lead Nitrate.
From brainly.com
Balance the chemical reaction using an atom inventory. What is the Lead (Ii) Nitrate And Lead Nitrate This compound is highly soluble in water Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. Lead(ii) nitrate is toxic and must be handled with care. N 2 o 6 pb. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. It consists of lead and nitrate ions and. Lead (Ii) Nitrate And Lead Nitrate.
From sincerechemical01.en.made-in-china.com
Chemical HighPurity Raw Material Lead (II) Nitrate China Lead (II Lead (Ii) Nitrate And Lead Nitrate It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Lead(ii) nitrate is toxic and must be handled with care. This compound is highly soluble in water Lead nitrate is also known as lead(ii) nitrate. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. It is an oxidizing agent and. Lead (Ii) Nitrate And Lead Nitrate.
From www.teachoo.com
MCQ Reema took 5ml of Lead Nitrate solution in a beaker and added ap Lead (Ii) Nitrate And Lead Nitrate Because of the insolubility of. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It is an oxidizing agent and is used to make other lead. N 2 o 6 pb. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. It is a chemical. Lead (Ii) Nitrate And Lead Nitrate.
From socratic.org
How do you write the the reaction of lead(II) nitrate (aq) with sodium Lead (Ii) Nitrate And Lead Nitrate This compound is highly soluble in water Because of the insolubility of. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Lead(ii) nitrate is toxic and must be handled with care. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It is a chemical compound with the formula pb(no₃)₂.. Lead (Ii) Nitrate And Lead Nitrate.
From www.youtube.com
007 Potassium Iodide + Lead (II) Nitrate = Lead (II) Iodide Lead (Ii) Nitrate And Lead Nitrate It is a chemical compound with the formula pb(no₃)₂. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. This compound is highly soluble in water Because of the insolubility of. Lead nitrate is also known as lead(ii) nitrate. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii). Lead (Ii) Nitrate And Lead Nitrate.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Lead (Ii) Nitrate And Lead Nitrate Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. Lead(ii) nitrate is toxic and must be handled with care. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. N 2 o 6 pb. This compound is highly soluble in water Lead nitrate is also known as lead(ii). Lead (Ii) Nitrate And Lead Nitrate.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Lead (Ii) Nitrate And Lead Nitrate It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. This compound is highly soluble in water It is an oxidizing agent and is used to make other lead. Lead(ii) nitrate is toxic and must be handled with care. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide. Lead (Ii) Nitrate And Lead Nitrate.
From picklifestyles.blogspot.com
Lead II Nitrate Reaction With Potassium Iodide Pb(NO3)2 Lifestyle News Lead (Ii) Nitrate And Lead Nitrate It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Lead nitrate is also known as lead(ii) nitrate. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. It is a chemical compound with the formula pb(no₃)₂. Since around the year 2000, lead(ii) nitrate has begun to. Lead (Ii) Nitrate And Lead Nitrate.
From www.chegg.com
Solved Solutions of lead(II) nitrate and barium chloride are Lead (Ii) Nitrate And Lead Nitrate It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It is an oxidizing agent and is used to make other lead. Lead nitrate is also known as lead(ii) nitrate. Since around the year 2000, lead(ii) nitrate has begun to be. Lead (Ii) Nitrate And Lead Nitrate.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead(II) nitrate reacts Lead (Ii) Nitrate And Lead Nitrate It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Lead(ii) nitrate is toxic and must be handled with care. N 2 o 6 pb. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate.. Lead (Ii) Nitrate And Lead Nitrate.
From brainly.in
Sodium chloride solution is added to lead nitrate solution. Also write Lead (Ii) Nitrate And Lead Nitrate It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. This compound is highly soluble in water It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. It describes the reactions. Lead (Ii) Nitrate And Lead Nitrate.
From socratic.org
Write the balanced chemical reacton between 20.0mL of .10M lead(II Lead (Ii) Nitrate And Lead Nitrate Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. Lead(ii) nitrate is toxic and must be handled with care. Because of the insolubility of. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. N 2 o 6 pb. It is an oxidizing agent and. Lead (Ii) Nitrate And Lead Nitrate.
From www.showme.com
Zinc and lead (II) nitrate react to form zinc nitrate and lead LHS Lead (Ii) Nitrate And Lead Nitrate It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It is a chemical compound with the formula pb(no₃)₂. It is an oxidizing agent and is used to make other lead. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Lead nitrate is a nitrate of lead produced synthetically by. Lead (Ii) Nitrate And Lead Nitrate.
From www.youtube.com
Predict the Products Pb(NO3)2 + KI Lead (II) Nitrate + Potassium Lead (Ii) Nitrate And Lead Nitrate Lead(ii) nitrate is toxic and must be handled with care. This compound is highly soluble in water It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Lead nitrate is also known as lead(ii) nitrate. N 2 o 6 pb. Because of the insolubility of. Since around the year 2000, lead(ii) nitrate has begun to be. Lead (Ii) Nitrate And Lead Nitrate.
From www.toppr.com
Identify the type of reactions taking place in each of the following Lead (Ii) Nitrate And Lead Nitrate It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. Because of the insolubility of. N 2 o 6 pb. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Lead(ii) nitrate is toxic and. Lead (Ii) Nitrate And Lead Nitrate.
From www.slideshare.net
Chemistry Lead (Ii) Nitrate And Lead Nitrate It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. N 2 o 6 pb. It is a chemical compound with the formula pb(no₃)₂. Because of the insolubility of. Lead nitrate is also known as lead(ii) nitrate. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid.. Lead (Ii) Nitrate And Lead Nitrate.
From www.chegg.com
Solved 1) Zinc and lead (II) nitrate react to form zinc Lead (Ii) Nitrate And Lead Nitrate It is an oxidizing agent and is used to make other lead. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Because of the insolubility of. This compound is highly soluble in water Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. Lead nitrate is a nitrate of lead. Lead (Ii) Nitrate And Lead Nitrate.
From www.slideshare.net
Precipitation react2 Lead (Ii) Nitrate And Lead Nitrate Because of the insolubility of. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. It is a chemical compound with the formula pb(no₃)₂. It is an oxidizing agent and is used to make other lead. Since around the year 2000,. Lead (Ii) Nitrate And Lead Nitrate.
From www.slideshare.net
Precipitation react2 Lead (Ii) Nitrate And Lead Nitrate N 2 o 6 pb. It is an oxidizing agent and is used to make other lead. This compound is highly soluble in water It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Lead(ii) nitrate is toxic and must be handled with care. Because of the insolubility of. It consists of lead and nitrate. Lead (Ii) Nitrate And Lead Nitrate.
From www.youtube.com
How to Balance Pb(NO3)2 + NaBr = PbBr2 + NaNO3 Lead (II) nitrate Lead (Ii) Nitrate And Lead Nitrate It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. Lead nitrate is also known as lead(ii) nitrate. It is an oxidizing agent and is used to make other lead. Lead(ii) nitrate is toxic and must be handled with care.. Lead (Ii) Nitrate And Lead Nitrate.
From www.slideserve.com
PPT 3. Solutions of sodium iodide and lead nitrate are mixed. I Lead (Ii) Nitrate And Lead Nitrate N 2 o 6 pb. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. It is a chemical compound with the formula pb(no₃)₂. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid.. Lead (Ii) Nitrate And Lead Nitrate.
From ar.inspiredpencil.com
Lead Ii Nitrate Solution Lead (Ii) Nitrate And Lead Nitrate Lead(ii) nitrate is toxic and must be handled with care. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. It is an oxidizing agent and is used to make other lead. This compound is highly soluble in water. Lead (Ii) Nitrate And Lead Nitrate.
From www.chegg.com
Solved If 1 mole of lead (II) nitrate reacts with 1 mole of Lead (Ii) Nitrate And Lead Nitrate Because of the insolubility of. Lead nitrate is also known as lead(ii) nitrate. Lead(ii) nitrate is toxic and must be handled with care. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. N 2. Lead (Ii) Nitrate And Lead Nitrate.
From molekula.com
Purchase Lead (II) nitrate [10099748] online • Catalog • Molekula Group Lead (Ii) Nitrate And Lead Nitrate It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Lead(ii) nitrate is toxic and must be handled with care. N 2 o 6. Lead (Ii) Nitrate And Lead Nitrate.
From www.slideshare.net
Preciptation reactions 1 Lead (Ii) Nitrate And Lead Nitrate This compound is highly soluble in water Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It is an oxidizing agent and is used to make other lead. Lead(ii) nitrate is toxic and must be handled with care. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Lead. Lead (Ii) Nitrate And Lead Nitrate.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Sodium Carbonate YouTube Lead (Ii) Nitrate And Lead Nitrate It is a chemical compound with the formula pb(no₃)₂. It is an oxidizing agent and is used to make other lead. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. N 2 o 6 pb. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric. Lead (Ii) Nitrate And Lead Nitrate.
From www.youtube.com
Double displacement NaCl + Pb(NO3)2 Sodium chloride + Lead(ii Lead (Ii) Nitrate And Lead Nitrate Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Lead(ii) nitrate is toxic and must be handled with care. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It is an oxidizing agent and is used to make other lead. It describes the formation. Lead (Ii) Nitrate And Lead Nitrate.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Potassium Iodide YouTube Lead (Ii) Nitrate And Lead Nitrate This compound is highly soluble in water It is an oxidizing agent and is used to make other lead. It is a chemical compound with the formula pb(no₃)₂. Lead nitrate is also known as lead(ii) nitrate. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It describes the formation of lead(ii) hydroxide, lead(ii) chloride,. Lead (Ii) Nitrate And Lead Nitrate.
From www.youtube.com
lead (II) nitrate And Sodium Iodide Make Lead (II) iodide And sodium Lead (Ii) Nitrate And Lead Nitrate It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. N 2 o 6 pb. Lead nitrate is also known as lead(ii) nitrate. Lead(ii) nitrate is toxic and must be handled with care. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulphate. Because of the insolubility of. Since. Lead (Ii) Nitrate And Lead Nitrate.
From alannahminhardy.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide AlannahminHardy Lead (Ii) Nitrate And Lead Nitrate Because of the insolubility of. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. Lead nitrate is also known as. Lead (Ii) Nitrate And Lead Nitrate.
From sincerechemical01.en.made-in-china.com
High Quality Lead (II) Nitrate, Focusing on Quality Lead (II) Nitrate Lead (Ii) Nitrate And Lead Nitrate Lead nitrate is also known as lead(ii) nitrate. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. It is. Lead (Ii) Nitrate And Lead Nitrate.
From lab.honeywell.com
Lead(II) nitrate 228621 Honeywell Research Chemicals Lead (Ii) Nitrate And Lead Nitrate Lead nitrate is also known as lead(ii) nitrate. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. N 2 o 6 pb. It consists of lead and nitrate ions and appears as a colorless or white crystalline solid. Because of the insolubility of. It describes the reactions to form lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide. Lead (Ii) Nitrate And Lead Nitrate.
From wayneferscarlson.blogspot.com
Is Lead Nitrate Ionic or Covalent Lead (Ii) Nitrate And Lead Nitrate N 2 o 6 pb. Since around the year 2000, lead(ii) nitrate has begun to be used in gold cyanidation. Lead nitrate is a nitrate of lead produced synthetically by dissolving metallic lead or lead(ii) oxide in nitric acid. Lead nitrate is also known as lead(ii) nitrate. This compound is highly soluble in water It is a chemical compound with. Lead (Ii) Nitrate And Lead Nitrate.