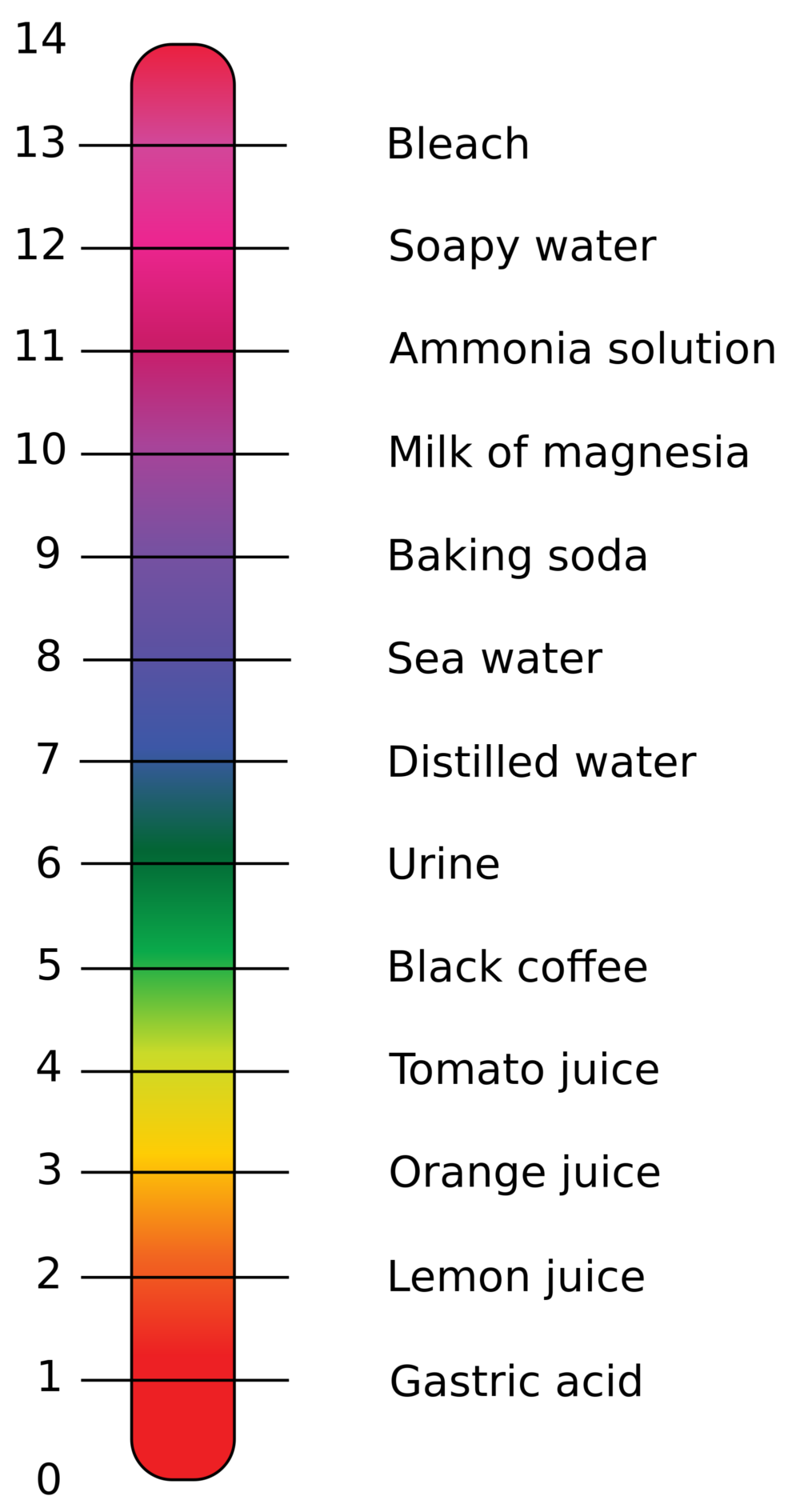
Examples Of Liquid Bases . Bases have properties that mostly contrast with those of acids. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Weak acids include acetic acid (like in vinegar) and formic acid. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. They are commonly found in household items like baking soda and soap. Here is a list of common household acids and bases, a look at the specific acids and bases they contain, and a list of chemical that are neither acids nor bases. Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Aqueous solutions of bases are also electrolytes. It is a white solid ionic. Bases can be either strong or. Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). Examples of strong bases are sodium hydroxide (naoh) and potassium hydroxide (koh). Bases are substances that taste bitter and feel slippery when dissolved in water.
from pluspng.com
They are commonly found in household items like baking soda and soap. Bases can be either strong or. Here is a list of common household acids and bases, a look at the specific acids and bases they contain, and a list of chemical that are neither acids nor bases. Weak acids include acetic acid (like in vinegar) and formic acid. Examples of strong bases are sodium hydroxide (naoh) and potassium hydroxide (koh). Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. It is a white solid ionic. Bases are substances that taste bitter and feel slippery when dissolved in water. Aqueous solutions of bases are also electrolytes.
Collection of Acid And Base PNG. PlusPNG
Examples Of Liquid Bases Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Bases have properties that mostly contrast with those of acids. Examples of strong bases are sodium hydroxide (naoh) and potassium hydroxide (koh). Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Bases are substances that taste bitter and feel slippery when dissolved in water. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Weak acids include acetic acid (like in vinegar) and formic acid. Aqueous solutions of bases are also electrolytes. Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). Here is a list of common household acids and bases, a look at the specific acids and bases they contain, and a list of chemical that are neither acids nor bases. Bases can be either strong or. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. It is a white solid ionic. They are commonly found in household items like baking soda and soap.
From www.youtube.com
U11L1 Facts on Acids and Bases YouTube Examples Of Liquid Bases Aqueous solutions of bases are also electrolytes. They are commonly found in household items like baking soda and soap. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Weak acids include acetic acid (like in vinegar) and formic acid. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt. Examples Of Liquid Bases.
From webmis.highland.cc.il.us
Aqueous Solutions Examples Of Liquid Bases Bases are substances that taste bitter and feel slippery when dissolved in water. Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Aqueous solutions of bases. Examples Of Liquid Bases.
From byjus.com
Difference between Acid and Base Differences btw Acid & Base in Examples Of Liquid Bases Here is a list of common household acids and bases, a look at the specific acids and bases they contain, and a list of chemical that are neither acids nor bases. Bases can be either strong or. It is a white solid ionic. Weak acids include acetic acid (like in vinegar) and formic acid. Bases have properties that mostly contrast. Examples Of Liquid Bases.
From www.slideserve.com
PPT Base Liquids PowerPoint Presentation, free download ID3224381 Examples Of Liquid Bases Bases are substances that taste bitter and feel slippery when dissolved in water. Weak acids include acetic acid (like in vinegar) and formic acid. Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Bases can be either strong or. Here is a list of common household acids and bases, a look at the specific acids and. Examples Of Liquid Bases.
From www.preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories Examples Of Liquid Bases Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Examples of strong bases are sodium hydroxide (naoh) and potassium hydroxide (koh). It is a white solid ionic. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Weak acids include acetic acid (like in vinegar) and formic acid. Bases can. Examples Of Liquid Bases.
From kidspressmagazine.com
Acids and Bases Examples Of Liquid Bases Weak acids include acetic acid (like in vinegar) and formic acid. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. It is a white solid ionic. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. They are commonly found in household items like baking soda. Examples Of Liquid Bases.
From www.slideserve.com
PPT Corrosive Materials PowerPoint Presentation, free download ID Examples Of Liquid Bases Here is a list of common household acids and bases, a look at the specific acids and bases they contain, and a list of chemical that are neither acids nor bases. They are commonly found in household items like baking soda and soap. Weak acids include acetic acid (like in vinegar) and formic acid. Examples of bases are the hydroxides. Examples Of Liquid Bases.
From www.pinterest.com
Liquid Bases That You Can Use To Make Your Smoothies Tiny Kitchen Examples Of Liquid Bases It is a white solid ionic. Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Bases have properties that mostly contrast with those of acids. Examples. Examples Of Liquid Bases.
From studylib.net
Acids and Bases Examples Of Liquid Bases Bases are substances that taste bitter and feel slippery when dissolved in water. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Aqueous solutions of bases are also electrolytes. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Examples of strong bases are sodium hydroxide (naoh). Examples Of Liquid Bases.
From praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Examples Of Liquid Bases Examples of strong bases are sodium hydroxide (naoh) and potassium hydroxide (koh). Bases have properties that mostly contrast with those of acids. Weak acids include acetic acid (like in vinegar) and formic acid. Aqueous solutions of bases are also electrolytes. It is a white solid ionic. Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. They. Examples Of Liquid Bases.
From www.exampleslab.com
20 Examples of Chemical Bases Examples Lab Examples Of Liquid Bases Weak acids include acetic acid (like in vinegar) and formic acid. Bases can be either strong or. It is a white solid ionic. Bases are substances that taste bitter and feel slippery when dissolved in water. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Examples of bases are the hydroxides of. Examples Of Liquid Bases.
From www.vecteezy.com
The chart shows the Acidic Neutral and Alkaline pH of various liquids Examples Of Liquid Bases It is a white solid ionic. Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). They are commonly found in household items like baking soda and soap. Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Bases have. Examples Of Liquid Bases.
From www.sciencephoto.com
Common Household Acids and Bases Stock Image C030/7537 Science Examples Of Liquid Bases Examples of strong bases are sodium hydroxide (naoh) and potassium hydroxide (koh). Here is a list of common household acids and bases, a look at the specific acids and bases they contain, and a list of chemical that are neither acids nor bases. It is a white solid ionic. Bases are substances that taste bitter and feel slippery when dissolved. Examples Of Liquid Bases.
From www.sssi.in
Acids, Bases, and Salts Key Concepts and Properties Explained Examples Of Liquid Bases Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). They are commonly found in household items like baking soda and soap. Examples of strong bases are sodium hydroxide (naoh) and potassium hydroxide (koh). It is a white solid ionic. Bases are substances. Examples Of Liquid Bases.
From simplelivingcreativelearning.com
Acid or Base Experiment Simple Living. Creative Learning Examples Of Liquid Bases Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Bases have properties that mostly contrast with those of acids. Weak acids include acetic acid (like in vinegar) and formic acid. Bases can be either strong or. They are commonly found in household items like baking soda and soap. Acids and bases undergo a chemical reaction or. Examples Of Liquid Bases.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo Examples Of Liquid Bases Examples of strong bases are sodium hydroxide (naoh) and potassium hydroxide (koh). Aqueous solutions of bases are also electrolytes. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Weak acids include acetic acid (like in vinegar) and formic acid. Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium,. Examples Of Liquid Bases.
From www.thoughtco.com
Examples of Chemical Reactions in Everyday Life Examples Of Liquid Bases Examples of strong bases are sodium hydroxide (naoh) and potassium hydroxide (koh). Bases are substances that taste bitter and feel slippery when dissolved in water. Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. They are commonly found in household items like baking soda and soap. Aqueous solutions of bases are also electrolytes. Bases have properties. Examples Of Liquid Bases.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Examples Of Liquid Bases Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Weak acids include acetic acid (like in vinegar) and formic acid. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. It is a white solid ionic. They are commonly found in household items like baking soda and soap. Examples of. Examples Of Liquid Bases.
From www.haikudeck.com
Liquid Properties by Madison Radtke Examples Of Liquid Bases Here is a list of common household acids and bases, a look at the specific acids and bases they contain, and a list of chemical that are neither acids nor bases. Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Bases can be either strong or. Aqueous solutions of bases are also electrolytes. Acids and bases. Examples Of Liquid Bases.
From www.dreamstime.com
Scale of Ph Value for Acid and Alkaline Solutions Stock Vector Examples Of Liquid Bases Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Weak acids include acetic acid (like in vinegar) and formic acid. Here is a list of. Examples Of Liquid Bases.
From pluspng.com
Collection of Acid And Base PNG. PlusPNG Examples Of Liquid Bases It is a white solid ionic. Bases can be either strong or. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Weak acids include. Examples Of Liquid Bases.
From www.youtube.com
Mixing Strong Concentrated Acid and Base YouTube Examples Of Liquid Bases Weak acids include acetic acid (like in vinegar) and formic acid. They are commonly found in household items like baking soda and soap. Here is a list of common household acids and bases, a look at the specific acids and bases they contain, and a list of chemical that are neither acids nor bases. Bases can be either strong or.. Examples Of Liquid Bases.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Examples Of Liquid Bases Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). Here is a list of common household acids and bases, a look at the specific acids and bases they contain, and a list of chemical that are neither acids nor bases. It is. Examples Of Liquid Bases.
From ar.inspiredpencil.com
Examples Of Bases Examples Of Liquid Bases Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Aqueous solutions of bases are also electrolytes. Weak acids include acetic acid (like in vinegar) and formic acid. Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives. Examples Of Liquid Bases.
From www.slideserve.com
PPT Common Bases PowerPoint Presentation, free download ID2782494 Examples Of Liquid Bases Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). It is a white solid ionic. Aqueous solutions of bases are also electrolytes. Bases can be either strong or. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt. Examples Of Liquid Bases.
From sciencenotes.org
Liquid Definition Examples of Liquids Examples Of Liquid Bases Bases are substances that taste bitter and feel slippery when dissolved in water. Weak acids include acetic acid (like in vinegar) and formic acid. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. They are commonly found in household items like baking soda and soap. Baking soda ( sodium bicarbonate ) and ammonia. Examples Of Liquid Bases.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo Examples Of Liquid Bases Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. They are commonly found in household items like baking soda and soap. Weak acids include acetic acid (like in vinegar) and formic acid. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Aqueous solutions of bases are. Examples Of Liquid Bases.
From fphoto.photoshelter.com
science chemistry acid base household products Fundamental Examples Of Liquid Bases Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Bases are substances that taste bitter and feel slippery when dissolved in water. Aqueous solutions of bases are also electrolytes. It is a white solid ionic. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Examples of. Examples Of Liquid Bases.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2789112 Examples Of Liquid Bases Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. They are commonly found in household items like baking soda and soap. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.). Examples Of Liquid Bases.
From www.thinglink.com
Bases are defined as a substance that produces hydroxide Examples Of Liquid Bases Aqueous solutions of bases are also electrolytes. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. They are commonly found in household items like baking soda and soap. Bases can be either strong or. Here is a list of common household acids and bases, a look at the specific acids and bases they. Examples Of Liquid Bases.
From philschatz.com
AcidBase Balance · Anatomy and Physiology Examples Of Liquid Bases Aqueous solutions of bases are also electrolytes. Weak acids include acetic acid (like in vinegar) and formic acid. It is a white solid ionic. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Bases are substances that taste bitter and feel slippery when dissolved in water. Bases have properties that mostly contrast with. Examples Of Liquid Bases.
From slideplayer.com
Chemical Reactions. ppt download Examples Of Liquid Bases Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Bases are substances that taste bitter and feel slippery when dissolved in water. Examples of bases are the hydroxides of the alkali and alkaline earth metals (sodium, calcium, etc.) and the water solutions of ammonia or its organic derivatives (amines). Weak acids include acetic. Examples Of Liquid Bases.
From www.teachoo.com
Uses of Base 5+ Examples Chemistry Teachoo Teachoo Questions Examples Of Liquid Bases Bases are substances that taste bitter and feel slippery when dissolved in water. They are commonly found in household items like baking soda and soap. Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with formula naoh. Weak acids include acetic acid (like in vinegar) and formic acid. Here is a list of common household acids. Examples Of Liquid Bases.
From www.youtube.com
3A 14.2 and 14.3 Acids & Bases Properties & Examples YouTube Examples Of Liquid Bases Bases are substances that taste bitter and feel slippery when dissolved in water. It is a white solid ionic. They are commonly found in household items like baking soda and soap. Baking soda ( sodium bicarbonate ) and ammonia are examples of weak bases. Weak acids include acetic acid (like in vinegar) and formic acid. Bases have properties that mostly. Examples Of Liquid Bases.
From oerpub.github.io
This figure shows four beakers containing different liquids. Examples Of Liquid Bases It is a white solid ionic. They are commonly found in household items like baking soda and soap. Bases are substances that taste bitter and feel slippery when dissolved in water. Bases have properties that mostly contrast with those of acids. Acids and bases undergo a chemical reaction or neutralize each other, forming a salt and water. Sodium hydroxide, also. Examples Of Liquid Bases.