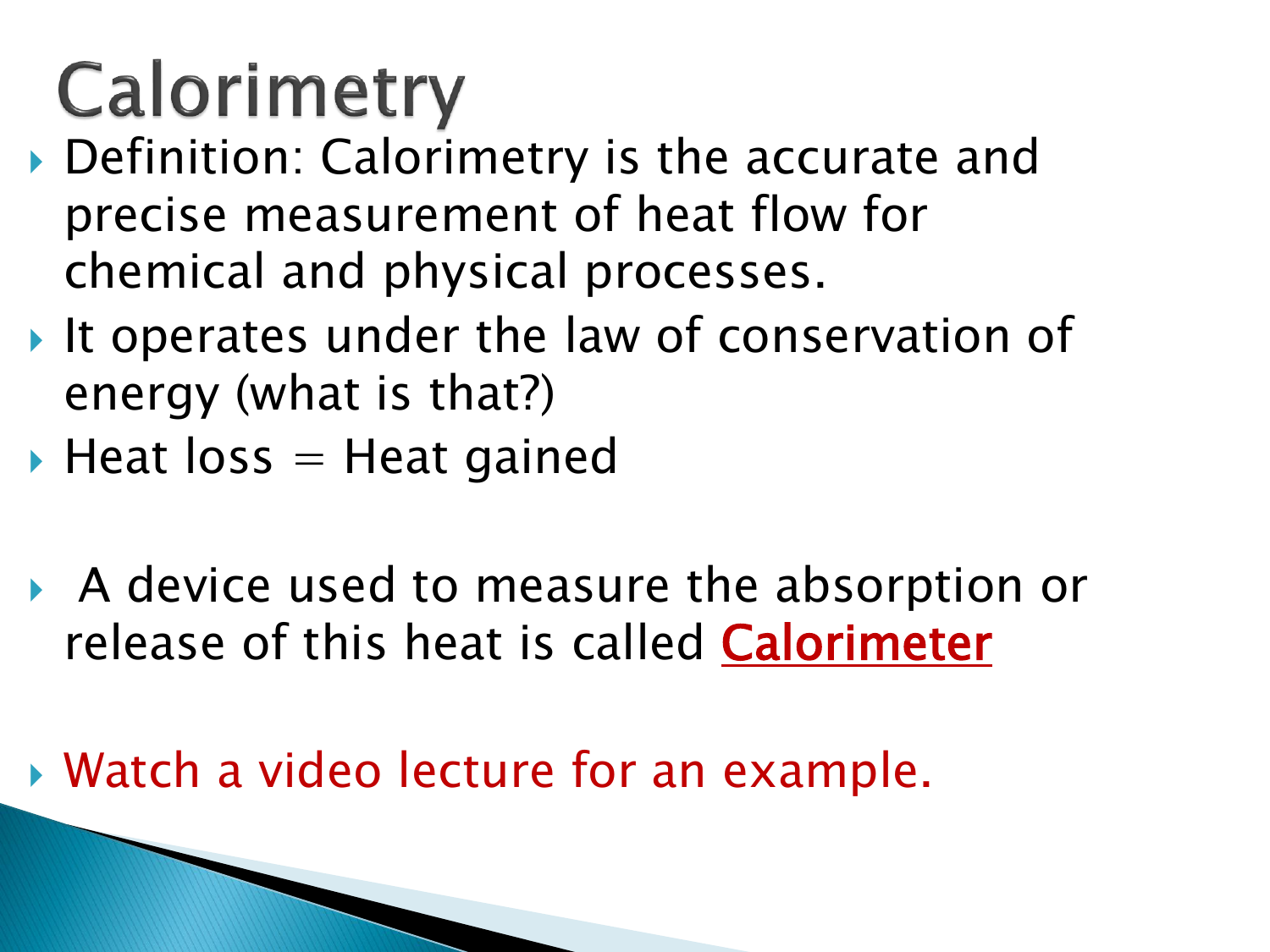
Calorimeter Value Meaning . calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. We need to find the difference between the heat lost by the hot water when it droped. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.
from studylib.net
a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We need to find the difference between the heat lost by the hot water when it droped. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the.
Calorimetry
Calorimeter Value Meaning what is the calorimeter constant? It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. We need to find the difference between the heat lost by the hot water when it droped. what is the calorimeter constant? calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Calorimeter Value Meaning what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We need to find the difference between the heat lost by the hot water when it droped. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure. Calorimeter Value Meaning.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimeter Value Meaning calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved. Calorimeter Value Meaning.
From www.surfacesciencewestern.com
Differential Scanning Calorimetry (DSC) Surface Science Western Calorimeter Value Meaning calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. what is the calorimeter constant? It uses devices called calorimeters, which measure the. We need to find the difference between the heat lost by the hot water when it droped. a calorimeter is a device used to measure the amount of heat involved. Calorimeter Value Meaning.
From evulpo.com
evulpo Calorimetry Calorimeter Value Meaning It uses devices called calorimeters, which measure the. We need to find the difference between the heat lost by the hot water when it droped. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is an instrument that has a thermally isolated compartment that is designed. Calorimeter Value Meaning.
From www.researchgate.net
(a) Differential scanning calorimetry (DSC) first heating scans at 10 Calorimeter Value Meaning a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure. Calorimeter Value Meaning.
From socratic.org
What is the change in internal energy of reaction when "0.721 g" of Calorimeter Value Meaning calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used. Calorimeter Value Meaning.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Value Meaning We need to find the difference between the heat lost by the hot water when it droped. what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. Calorimeter Value Meaning.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Value Meaning what is the calorimeter constant? calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. We need to find the difference between the heat lost by the hot water when it droped. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a. Calorimeter Value Meaning.
From guernseydonkey.com
How is the Caloric Value of Food Measured? Calorimeter Value Meaning what is the calorimeter constant? It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. We need to find the difference between the heat lost by the hot water when it droped. a calorimeter is a device used to measure the amount of heat involved. Calorimeter Value Meaning.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimeter Value Meaning a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. what is the calorimeter constant? calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. . Calorimeter Value Meaning.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Calorimeter Value Meaning We need to find the difference between the heat lost by the hot water when it droped. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter. Calorimeter Value Meaning.
From studylib.net
Calorimetry Calorimeter Value Meaning It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. what is the calorimeter constant? We need to find the difference between the heat lost by the hot water when it droped. a calorimeter is an instrument that has a thermally isolated compartment that is. Calorimeter Value Meaning.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Value Meaning We need to find the difference between the heat lost by the hot water when it droped. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.. Calorimeter Value Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Value Meaning calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. We need to find the difference between the heat lost by the hot water when it droped. what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimeter Value Meaning.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Value Meaning a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. what is the calorimeter constant? calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. We need to find the difference between the heat lost by the hot water when it droped. calorimetry. Calorimeter Value Meaning.
From frankmccomb.info
Which Statement Describes How a Basic Coffee Cup Calorimeter Works? Calorimeter Value Meaning We need to find the difference between the heat lost by the hot water when it droped. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. a calorimeter is a. Calorimeter Value Meaning.
From www.youtube.com
How to solve problems on GCV and NCV of a fuel sample for the bomb Calorimeter Value Meaning We need to find the difference between the heat lost by the hot water when it droped. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in. Calorimeter Value Meaning.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Calorimeter Value Meaning calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. what is the calorimeter constant? calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. We need to find the difference between the heat lost by the hot water when it droped. a. Calorimeter Value Meaning.
From www.youtube.com
Determination of calorific value of solid/liquid fuel using Bomb Calorimeter Value Meaning what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is an instrument that has a thermally isolated compartment that is. Calorimeter Value Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Value Meaning We need to find the difference between the heat lost by the hot water when it droped. what is the calorimeter constant? calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. It uses devices called. Calorimeter Value Meaning.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Value Meaning It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We need to find the difference between the heat lost by the hot water when it droped. what is the calorimeter constant? calorimetry is the set of techniques used to. Calorimeter Value Meaning.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Value Meaning a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is an. Calorimeter Value Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimeter Value Meaning a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. calorimetry. Calorimeter Value Meaning.
From chemistry.stackexchange.com
thermodynamics Heat capacity of calorimeter is negative? Chemistry Calorimeter Value Meaning We need to find the difference between the heat lost by the hot water when it droped. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set. Calorimeter Value Meaning.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimeter Value Meaning a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. We need to find the difference between the heat lost by the hot water when it droped. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a. Calorimeter Value Meaning.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimeter Value Meaning It uses devices called calorimeters, which measure the. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat. Calorimeter Value Meaning.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Value Meaning a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the. what is the calorimeter constant? calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is a field of thermochemistry that measures the amount. Calorimeter Value Meaning.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter Value Meaning It uses devices called calorimeters, which measure the. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy changes during chemical. Calorimeter Value Meaning.
From www.youtube.com
Calorimeter meaning of Calorimeter YouTube Calorimeter Value Meaning calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry. Calorimeter Value Meaning.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Value Meaning We need to find the difference between the heat lost by the hot water when it droped. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is an instrument that has. Calorimeter Value Meaning.
From slideplayer.com
Calorimetry. ppt download Calorimeter Value Meaning We need to find the difference between the heat lost by the hot water when it droped. It uses devices called calorimeters, which measure the. what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to. Calorimeter Value Meaning.
From www.numerade.com
SOLVED Use the References to access important values if needed for Calorimeter Value Meaning a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. It uses. Calorimeter Value Meaning.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.1.2 Calorimetry Calorimeter Value Meaning It uses devices called calorimeters, which measure the. We need to find the difference between the heat lost by the hot water when it droped. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is an instrument that has a thermally isolated compartment that is designed. Calorimeter Value Meaning.
From www.studocu.com
Measurement of calorific value using bomb and Boys calorimeter Calorimeter Value Meaning a calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is a field of thermochemistry. Calorimeter Value Meaning.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimeter Value Meaning a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimeter Value Meaning.