Medical Device Standards Fda . Medical devices are products or equipment intended for a medical purpose. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. Exemptions from federal preemption of state and local medical device requirements: The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. In the european union (eu) they must undergo a conformity. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical.
from www.slideshare.net
Exemptions from federal preemption of state and local medical device requirements: This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. In the european union (eu) they must undergo a conformity. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. Medical devices are products or equipment intended for a medical purpose.
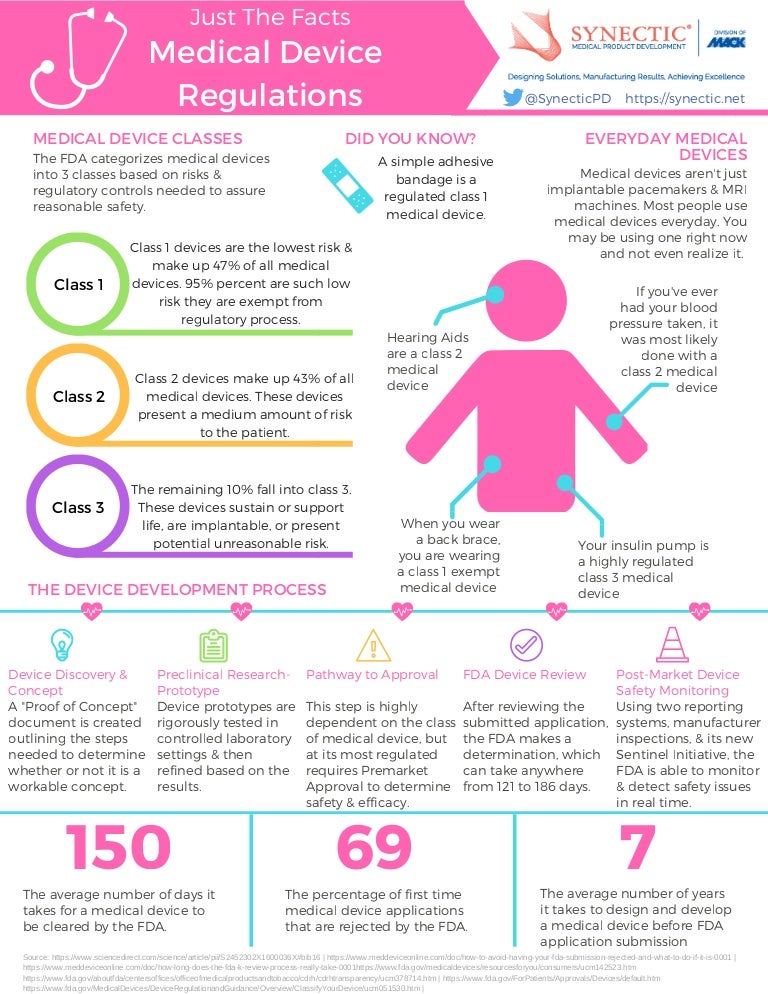
Medical Device FDA Regulations and Classifications infographic
Medical Device Standards Fda Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Exemptions from federal preemption of state and local medical device requirements: The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Standards Fda Exemptions from federal preemption of state and local medical device requirements: Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The division of standards and conformity assessment (dsca) seeks to promote. Medical Device Standards Fda.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Standards Fda The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. Exemptions from federal preemption of state and local medical device requirements: Medical devices are products or equipment intended for a medical purpose. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The fda is responsible. Medical Device Standards Fda.
From innolitics.com
2013 FDA Guidance Medical Device Classification Product Codes Medical Device Standards Fda The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. Exemptions. Medical Device Standards Fda.
From www.tailoredlabel.com
UDI Label Requirements For FDA Medical Device Labels TLP Medical Device Standards Fda In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. Exemptions from federal preemption of. Medical Device Standards Fda.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Medical Device Standards Fda This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. In the european union (eu) they must undergo a conformity. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. The fda is responsible for assuring medical devices available in the united states are safe and. Medical Device Standards Fda.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Medical Device Standards Fda This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. The. Medical Device Standards Fda.
From www.greenlight.guru
Ultimate Guide to ISO 13485 Quality Management System (QMS) for Medical Medical Device Standards Fda The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. In the european union (eu) they must undergo a conformity. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Exemptions from federal preemption of state and local medical device requirements: This. Medical Device Standards Fda.
From mavink.com
Mdr Classification Chart Medical Device Standards Fda The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Medical devices are products or equipment intended for a medical purpose. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. In the european union (eu) they must undergo a conformity. The. Medical Device Standards Fda.
From mavink.com
Fda Medical Device Classification Chart Medical Device Standards Fda Exemptions from federal preemption of state and local medical device requirements: In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. The division of standards and conformity assessment. Medical Device Standards Fda.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Medical Device Standards Fda Medical devices are products or equipment intended for a medical purpose. Exemptions from federal preemption of state and local medical device requirements: This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. In the european union. Medical Device Standards Fda.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Standards Fda Exemptions from federal preemption of state and local medical device requirements: Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. This document specifies a framework for the identification, and if necessary,. Medical Device Standards Fda.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Standards Fda The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Exemptions from federal preemption of state and local medical device requirements: Medical devices are products or equipment intended for a medical purpose. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and.. Medical Device Standards Fda.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Medical Device Standards Fda Exemptions from federal preemption of state and local medical device requirements: This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. Medical devices are products or equipment intended for a medical purpose. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. The fda is responsible. Medical Device Standards Fda.
From www.slideshare.net
US FDA medical device approval chart Emergo Group Medical Device Standards Fda The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Medical devices are products or equipment intended for a medical purpose. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The division of standards and conformity assessment (dsca) seeks to promote. Medical Device Standards Fda.
From mavink.com
Fda Medical Device Classification Chart Medical Device Standards Fda The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Exemptions from federal preemption of state and local medical device requirements: This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. Medical devices are products or equipment intended for a medical purpose.. Medical Device Standards Fda.
From www.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Device Standards Fda Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. This. Medical Device Standards Fda.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Medical Device Standards Fda Medical devices are products or equipment intended for a medical purpose. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. In the european union (eu) they must undergo a conformity. This. Medical Device Standards Fda.
From vem-medical.com
Medical Device Manufacturing Medical Device Standards Fda In the european union (eu) they must undergo a conformity. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. Medical devices are products or equipment intended for a medical purpose. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. The. Medical Device Standards Fda.
From operonstrategist.com
International Medical Device Standards ISO 13485, ISO 14971 Medical Device Standards Fda This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. In the european union (eu) they must undergo a conformity. Exemptions from federal preemption of state and local medical device requirements: The fda is responsible for. Medical Device Standards Fda.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Medical Device Standards Fda In the european union (eu) they must undergo a conformity. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Medical devices are products or equipment intended for a medical purpose. This. Medical Device Standards Fda.
From www.greenlight.guru
ISO Standards for Medical Devices Ultimate List & Overview Medical Device Standards Fda In the european union (eu) they must undergo a conformity. Exemptions from federal preemption of state and local medical device requirements: This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. The fda is responsible for. Medical Device Standards Fda.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Standards Fda In the european union (eu) they must undergo a conformity. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Medical devices are products or equipment intended for a medical purpose. Exemptions from federal preemption of state and local medical device requirements: This document specifies a framework for the. Medical Device Standards Fda.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Device Standards Fda This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Exemptions from federal preemption of state and local medical device requirements: The division of standards and conformity assessment (dsca) seeks to promote. Medical Device Standards Fda.
From www.slideserve.com
PPT Overview of FDA Device Regulations PowerPoint Presentation, free Medical Device Standards Fda The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. Exemptions from federal preemption of state and local medical device requirements: This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The fda is responsible for assuring medical devices available in the united states are safe. Medical Device Standards Fda.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Medical Device Standards Fda Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The fda is responsible for. Medical Device Standards Fda.
From rbccorp.com
What You Need to Know About The FDA’s Medical Device Cybersecurity Medical Device Standards Fda Exemptions from federal preemption of state and local medical device requirements: The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Medical devices are products or equipment intended for a medical purpose.. Medical Device Standards Fda.
From www.rimsys.io
De Novo classification process a beginner's guide Medical Device Standards Fda The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Exemptions from federal preemption of. Medical Device Standards Fda.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Standards Fda Medical devices are products or equipment intended for a medical purpose. Exemptions from federal preemption of state and local medical device requirements: This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product.. Medical Device Standards Fda.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Standards Fda Exemptions from federal preemption of state and local medical device requirements: The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. This document specifies a framework for the identification, and if necessary,. Medical Device Standards Fda.
From hackaday.io
FDA Product Code, 510(k), Medical Device Standards/Regulations Medical Device Standards Fda The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. In the european union (eu) they must undergo a conformity. Exemptions from federal preemption of state and local medical device requirements: The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Medical. Medical Device Standards Fda.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Standards Fda In the european union (eu) they must undergo a conformity. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. Exemptions from federal preemption of state and local medical device requirements: This. Medical Device Standards Fda.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Standards Fda Exemptions from federal preemption of state and local medical device requirements: Medical devices are products or equipment intended for a medical purpose. The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and.. Medical Device Standards Fda.
From www.rimsys.io
FDA listed, cleared, approved, granted what do these mean, and what’s Medical Device Standards Fda Exemptions from federal preemption of state and local medical device requirements: The fda is responsible for assuring medical devices available in the united states are safe and effective throughout their total product. In the european union (eu) they must undergo a conformity. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The. Medical Device Standards Fda.
From ramtechno.com
What Are the Three FDA Classes for Medical Devices? RAM Technologies Medical Device Standards Fda The division of standards and conformity assessment (dsca) seeks to promote patient safety, advance regulatory science, and. Exemptions from federal preemption of state and local medical device requirements: Medical devices are products or equipment intended for a medical purpose. This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. The fda is responsible. Medical Device Standards Fda.
From klanjwrju.blob.core.windows.net
Fda Medical Device Labeling Guidance at Michael Crawford blog Medical Device Standards Fda This document specifies a framework for the identification, and if necessary, quantification of constituents of a medical. Exemptions from federal preemption of state and local medical device requirements: Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. The division of standards and conformity assessment (dsca) seeks to promote. Medical Device Standards Fda.