Electrolysis For Metal . As far as the mechanism of the process is concerned,. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. It is not recommended for brass, aluminum, copper. this is a fantastic way to remove rust and oxidation from steel and iron. we have heard of the coating of one metal on another metal using the process of electrolysis. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. By harnessing the power of a.
from spmscience.blog.onlinetuition.com.my
by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. By harnessing the power of a. we have heard of the coating of one metal on another metal using the process of electrolysis. this is a fantastic way to remove rust and oxidation from steel and iron. It is not recommended for brass, aluminum, copper. As far as the mechanism of the process is concerned,. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis.
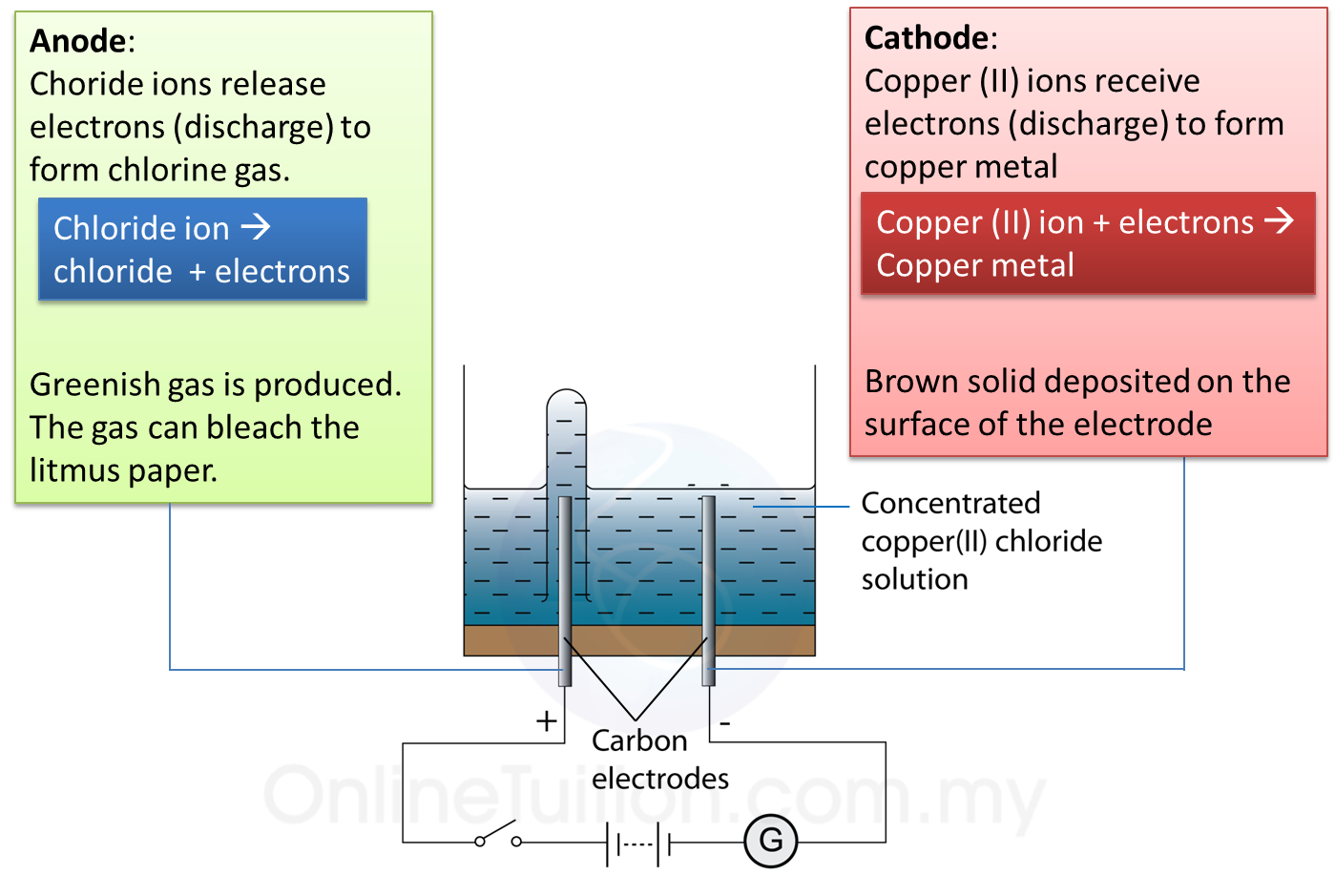
Electrolysis of Copper (II) Chloride Solution SPM Science
Electrolysis For Metal in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. this is a fantastic way to remove rust and oxidation from steel and iron. By harnessing the power of a. As far as the mechanism of the process is concerned,. It is not recommended for brass, aluminum, copper. by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. we have heard of the coating of one metal on another metal using the process of electrolysis. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath.
From www.youtube.com
Electrolysis of Copper Sulphate YouTube Electrolysis For Metal It is not recommended for brass, aluminum, copper. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. As far as the mechanism of the process is concerned,. we have heard of the coating of one metal on another metal using the process of electrolysis. electrolytic refining is. Electrolysis For Metal.
From www.researchgate.net
Scheme of the installation for the electrolysis of a metal powder of Electrolysis For Metal this is a fantastic way to remove rust and oxidation from steel and iron. As far as the mechanism of the process is concerned,. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis.. Electrolysis For Metal.
From www.researchgate.net
Schematic drawing of an aluminum electrolysis cell Download Electrolysis For Metal this is a fantastic way to remove rust and oxidation from steel and iron. By harnessing the power of a. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. in chemistry and. Electrolysis For Metal.
From keystagewiki.com
Extracting Metals by Electrolysis Key Stage Wiki Electrolysis For Metal By harnessing the power of a. this is a fantastic way to remove rust and oxidation from steel and iron. It is not recommended for brass, aluminum, copper. As far as the mechanism of the process is concerned,. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. we have. Electrolysis For Metal.
From courses.lumenlearning.com
Electrolysis Chemistry Electrolysis For Metal As far as the mechanism of the process is concerned,. By harnessing the power of a. It is not recommended for brass, aluminum, copper. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. . Electrolysis For Metal.
From electrolysis1.weebly.com
Electrolysis of Aluminium Oxide Electrolysis Electrolysis For Metal By harnessing the power of a. we have heard of the coating of one metal on another metal using the process of electrolysis. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. It is not recommended for brass, aluminum, copper. electrolysis is a reliable, cheap way to. Electrolysis For Metal.
From spmscience.blog.onlinetuition.com.my
Electrolysis of Copper (II) Chloride Solution SPM Science Electrolysis For Metal As far as the mechanism of the process is concerned,. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. By harnessing the power of a. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. It is not recommended for brass,. Electrolysis For Metal.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrolysis For Metal By harnessing the power of a. this is a fantastic way to remove rust and oxidation from steel and iron. we have heard of the coating of one metal on another metal using the process of electrolysis. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. As far as. Electrolysis For Metal.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis For Metal by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. As far as the mechanism of the process is concerned,. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. It is not recommended for brass, aluminum, copper. in chemistry. Electrolysis For Metal.
From saylordotorg.github.io
Electrolysis Electrolysis For Metal by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. As far as the mechanism of the process is concerned,. in chemistry and manufacturing, electrolysis is a technique that uses. Electrolysis For Metal.
From courses.lumenlearning.com
Occurrence and Preparation of the Representative Metals Chemistry Electrolysis For Metal It is not recommended for brass, aluminum, copper. this is a fantastic way to remove rust and oxidation from steel and iron. we have heard of the coating of one metal on another metal using the process of electrolysis. By harnessing the power of a. As far as the mechanism of the process is concerned,. by creating. Electrolysis For Metal.
From vectormine.com
Electrolysis chemical technique explanation for DC production outline Electrolysis For Metal we have heard of the coating of one metal on another metal using the process of electrolysis. As far as the mechanism of the process is concerned,. by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. By harnessing the power of a. electrolytic refining is a. Electrolysis For Metal.
From webmis.highland.cc.il.us
Electrolysis Electrolysis For Metal by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. It is not recommended for brass, aluminum, copper. electrolytic refining is a process of refining a metal (mainly. Electrolysis For Metal.
From revisechemistry.uk
Electrolysis Edexcel T3 revisechemistry.uk Electrolysis For Metal by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. It is not recommended for brass, aluminum, copper. this is a fantastic way to remove rust and oxidation from steel and iron. electrolytic refining is a process of refining a metal (mainly copper) by the process of. Electrolysis For Metal.
From courses.lumenlearning.com
Electrolysis Chemistry Electrolysis For Metal we have heard of the coating of one metal on another metal using the process of electrolysis. this is a fantastic way to remove rust and oxidation from steel and iron. It is not recommended for brass, aluminum, copper. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. in. Electrolysis For Metal.
From www.youtube.com
Using Electrolysis To Extract Metals (GCSE Chemistry) YouTube Electrolysis For Metal electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. By harnessing the power of a. It is not recommended for brass, aluminum, copper. this is a fantastic way to remove. Electrolysis For Metal.
From www.ggp-metal.com
The principle of electrolysis Electrolysis For Metal we have heard of the coating of one metal on another metal using the process of electrolysis. As far as the mechanism of the process is concerned,. by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. this is a fantastic way to remove rust and oxidation. Electrolysis For Metal.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis For Metal By harnessing the power of a. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. As far as the mechanism of the process is concerned,. It is not recommended for brass,. Electrolysis For Metal.
From chem2u.blogspot.co.uk
chem2U Electrolysis For Metal we have heard of the coating of one metal on another metal using the process of electrolysis. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. It is not recommended for brass, aluminum, copper. As far as the mechanism of the process is concerned,. by creating electrolysis,. Electrolysis For Metal.
From www.goodscience.com.au
Extraction of Metals Good Science Electrolysis For Metal It is not recommended for brass, aluminum, copper. by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. By harnessing the power of a. As far as the mechanism of the process. Electrolysis For Metal.
From www.youtube.com
Using Electrolysis to Extract Metals YouTube Electrolysis For Metal in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. It is not recommended for brass, aluminum, copper. By harnessing the power of a. As far as the mechanism of the process. Electrolysis For Metal.
From 2012books.lardbucket.org
Electrochemistry Electrolysis For Metal by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. this is a fantastic way to remove rust and oxidation from steel and iron. By harnessing the power of a. It is not recommended for brass, aluminum, copper. electrolytic refining is a process of refining a metal. Electrolysis For Metal.
From childhealthpolicy.vumc.org
😝 Electrolysis using copper electrodes. 0620 QR Dynamic Papers Electrolysis For Metal this is a fantastic way to remove rust and oxidation from steel and iron. By harnessing the power of a. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. by. Electrolysis For Metal.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Electrolysis For Metal in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. It is not recommended for brass, aluminum, copper. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. by creating electrolysis, we are using a positive electric charge (anodes) flowing through. Electrolysis For Metal.
From keystagewiki.com
Electrolysis Key Stage Wiki Electrolysis For Metal by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. this is a fantastic way to remove rust and oxidation from steel and iron. By harnessing the power of a. we have heard of the coating of one metal on another metal using the process of electrolysis.. Electrolysis For Metal.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Electrolysis For Metal in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. It is not recommended for brass, aluminum, copper. by creating electrolysis, we are using a positive electric charge (anodes) flowing through. Electrolysis For Metal.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrolysis For Metal electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. As far as the mechanism of the process is concerned,. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. we have heard of the coating of one metal on another metal. Electrolysis For Metal.
From uen.pressbooks.pub
Electrolysis Introductory Chemistry Electrolysis For Metal electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. As far as the mechanism of the process is concerned,. in chemistry and manufacturing, electrolysis is a technique that uses. Electrolysis For Metal.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis For Metal we have heard of the coating of one metal on another metal using the process of electrolysis. It is not recommended for brass, aluminum, copper. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to. Electrolysis For Metal.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Electrolysis Of Aluminium Oxide Video Electrolysis For Metal By harnessing the power of a. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. As far as the mechanism of the process is concerned,. electrolysis is a reliable, cheap way. Electrolysis For Metal.
From www.scribd.com
Worksheet Electrolysis PDF Chemical Compounds Physical Chemistry Electrolysis For Metal As far as the mechanism of the process is concerned,. By harnessing the power of a. this is a fantastic way to remove rust and oxidation from steel and iron. in chemistry and manufacturing, electrolysis is a technique that uses direct electric current (dc) to drive an otherwise non. electrolysis is a reliable, cheap way to remove. Electrolysis For Metal.
From www.numerade.com
Electrolysis of an alkaline earth metal chloride using a current of 5. Electrolysis For Metal As far as the mechanism of the process is concerned,. By harnessing the power of a. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. It is not recommended for brass, aluminum, copper. we have heard of the coating of one metal on another metal using the process of electrolysis. . Electrolysis For Metal.
From chemicalengineeringworld.com
Electrolysis Overview Chemical Engineering World Electrolysis For Metal by creating electrolysis, we are using a positive electric charge (anodes) flowing through a sacrificial piece of steel — i. this is a fantastic way to remove rust and oxidation from steel and iron. we have heard of the coating of one metal on another metal using the process of electrolysis. in chemistry and manufacturing, electrolysis. Electrolysis For Metal.
From chem1180.blogspot.com
CHEM 1180 19.919.11 Electrolysis of Molten SaltsStoichiometry of Electrolysis For Metal this is a fantastic way to remove rust and oxidation from steel and iron. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. electrolysis is a reliable, cheap way to remove rust from metal objects without hurting the material underneath. we have heard of the coating of one metal. Electrolysis For Metal.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Electrolysis For Metal It is not recommended for brass, aluminum, copper. electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. this is a fantastic way to remove rust and oxidation from steel and iron. As far as the mechanism of the process is concerned,. we have heard of the coating of one metal. Electrolysis For Metal.