Irb Oversight . Irbs play a critical role in the oversight of clinical research. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other.
from www.semanticscholar.org
This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. Irbs play a critical role in the oversight of clinical research. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other.
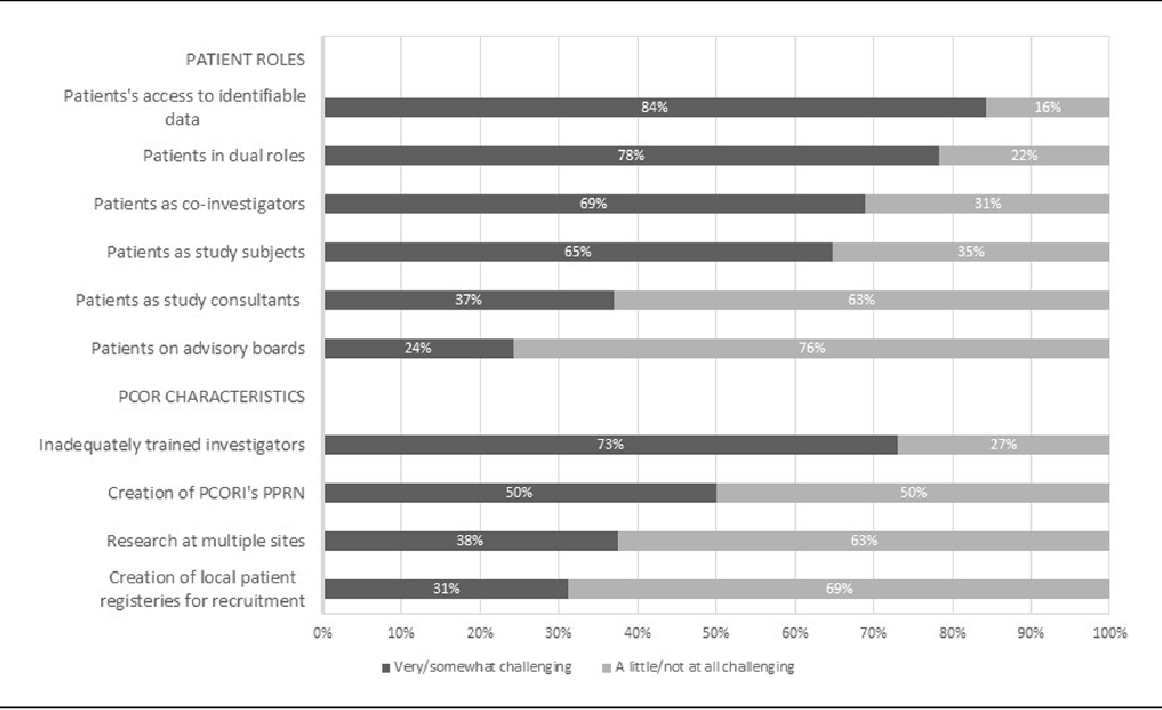
Figure 1 from IRB Oversight of PatientCentered Research A
Irb Oversight Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. Irbs play a critical role in the oversight of clinical research.
From scite.ai
The Harvard Catalyst Common Reciprocal IRB Reliance Agreement An Irb Oversight An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as. Irb Oversight.
From www.youtube.com
RCF Session 3 Diversity, Equity and Inclusion in IRB Review and Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. Irbs play a critical role in the oversight of clinical research. Under fda regulations, an. Irb Oversight.
From slideplayer.com
IRB Member Training. ppt download Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory. Irb Oversight.
From www.semanticscholar.org
Table 3 from IRB Oversight of PatientCentered Research A Irb Oversight An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. Irbs play a. Irb Oversight.
From www.slideserve.com
PPT Central IRBs Ceding IRB Oversight PowerPoint Presentation, free Irb Oversight This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight. Irb Oversight.
From www.semanticscholar.org
[PDF] A Framework for Navigating Institutional Review Board (IRB Irb Oversight Irbs play a critical role in the oversight of clinical research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. An institutional review board (irb) is the institutional entity charged with providing. Irb Oversight.
From www.pdffiller.com
Split IRB Oversight Agreement Doc Template pdfFiller Irb Oversight This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. The purpose of. Irb Oversight.
From www.cpp.edu
IRB Irb Oversight Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of. Irb Oversight.
From www.cureus.com
A Framework for Navigating Institutional Review Board (IRB) Oversight Irb Oversight This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This report describes. Irb Oversight.
From studylib.net
Ceding IRB Oversight Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. Irbs play a critical role in the oversight of clinical research. An institutional review. Irb Oversight.
From www.semanticscholar.org
Table 2 from IRB Oversight of PatientCentered Research A Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory. Irb Oversight.
From www.semanticscholar.org
Figure 1 from IRB Oversight of PatientCentered Research A Irb Oversight This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. An institutional review. Irb Oversight.
From www.slideserve.com
PPT Central IRBs Ceding IRB Oversight PowerPoint Presentation, free Irb Oversight This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. Under fda regulations, an institutional review board is group that has been formally designated to. Irb Oversight.
From www.tamiu.edu
Submission, Renewal, Closeout, and Other Forms Irb Oversight Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. Irbs play a critical role in the oversight of clinical research. This guidance is intended for institutions and institutional review boards. Irb Oversight.
From www.slideserve.com
PPT Central IRBs Ceding IRB Oversight PowerPoint Presentation, free Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. Under fda regulations, an institutional review board is group that has been formally designated to. Irb Oversight.
From www.advarra.com
Transfer of IRB Oversight During COVID19 Advarra Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight. Irb Oversight.
From www.semanticscholar.org
Table 1 from IRB Oversight of PatientCentered Research A Irb Oversight This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. Irbs play a critical role in the oversight of clinical research. Under fda regulations, an institutional review board is group that has. Irb Oversight.
From www.slideserve.com
PPT Research Matters An Introduction to IRBs and Research at Irb Oversight Irbs play a critical role in the oversight of clinical research. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This guidance is intended for institutions and institutional review boards. Irb Oversight.
From slideplayer.com
IRB Applications and Proposals ppt download Irb Oversight Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of. Irb Oversight.
From www.formsbank.com
28 Irb Form Templates free to download in PDF Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This report describes the composition of the irb market and examines ohrp and fda oversight. Irb Oversight.
From www.athreon.com
IRB Compliance and Ethical Research Transcription Outsourcing Irb Oversight This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. Irbs play a critical role in the oversight of clinical research. This guidance is intended. Irb Oversight.
From www.cpp.edu
IRB Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. An institutional review board (irb) is the institutional entity charged with providing ethical and. Irb Oversight.
From www.pdffiller.com
Fillable Online IRB LIMITED REVIEW FOR CIRB OVERSIGHT Cone Health Fax Irb Oversight An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants. Irb Oversight.
From www.researchgate.net
(PDF) A Framework for Navigating Institutional Review Board (IRB Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. This report describes the composition of the irb market and examines ohrp and fda. Irb Oversight.
From www.pdffiller.com
Advarra Statement of IRB Oversight Doc Template pdfFiller Irb Oversight This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. Irbs play a critical. Irb Oversight.
From www.slideserve.com
PPT Central IRBs Ceding IRB Oversight PowerPoint Presentation, free Irb Oversight Irbs play a critical role in the oversight of clinical research. Under fda regulations, an institutional review board is group that has been formally designated to review and monitor biomedical research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. The purpose of an irb review is to ensure that. Irb Oversight.
From www.cureus.com
Cureus A Framework for Navigating Institutional Review Board (IRB Irb Oversight Irbs play a critical role in the oversight of clinical research. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This report describes the composition of the irb market and examines ohrp. Irb Oversight.
From www.slideserve.com
PPT How to Navigate the IRB Process PowerPoint Presentation, free Irb Oversight An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. Irbs play a critical role in the oversight of clinical research. This guidance is intended. Irb Oversight.
From slideplayer.com
Introduction to Research Involving Human Subjects & the IRB at Florida Irb Oversight This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. Irbs play a critical role in the oversight of clinical research. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This guidance is intended for institutions and institutional review boards (irbs). Irb Oversight.
From www.slideserve.com
PPT IRB Responsibilities PowerPoint Presentation, free download ID Irb Oversight Irbs play a critical role in the oversight of clinical research. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. The purpose of an irb review is to ensure that appropriate steps. Irb Oversight.
From www.slideserve.com
PPT NIH Regional Seminar PowerPoint Presentation, free download ID Irb Oversight An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as. Irb Oversight.
From mrctcenter.org
IRB/EC Considerations for DCT Review The MultiRegional Clinical Irb Oversight This guidance is intended for institutions and institutional review boards (irbs) responsible for review and oversight of human. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. This report describes the composition of the irb market and examines ohrp and fda oversight of. Irb Oversight.
From www.semanticscholar.org
Figure 2 from IRB Oversight of PatientCentered Research A Irb Oversight An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. This report describes the composition of the irb market and examines ohrp and fda oversight. Irb Oversight.
From irb.upenn.edu
Penn IRB Levels of IRB Review Penn IRB Irb Oversight The purpose of an irb review is to ensure that appropriate steps are taken to protect the rights and welfare of participants as subjects of research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. Under fda regulations, an institutional review board is group that has been formally designated to. Irb Oversight.
From www.slideserve.com
PPT IRBMED and Chesapeake IRB PowerPoint Presentation, free download Irb Oversight Irbs play a critical role in the oversight of clinical research. This report describes the composition of the irb market and examines ohrp and fda oversight of irbs, among other. An institutional review board (irb) is the institutional entity charged with providing ethical and regulatory oversight of research involving. This guidance is intended for institutions and institutional review boards (irbs). Irb Oversight.