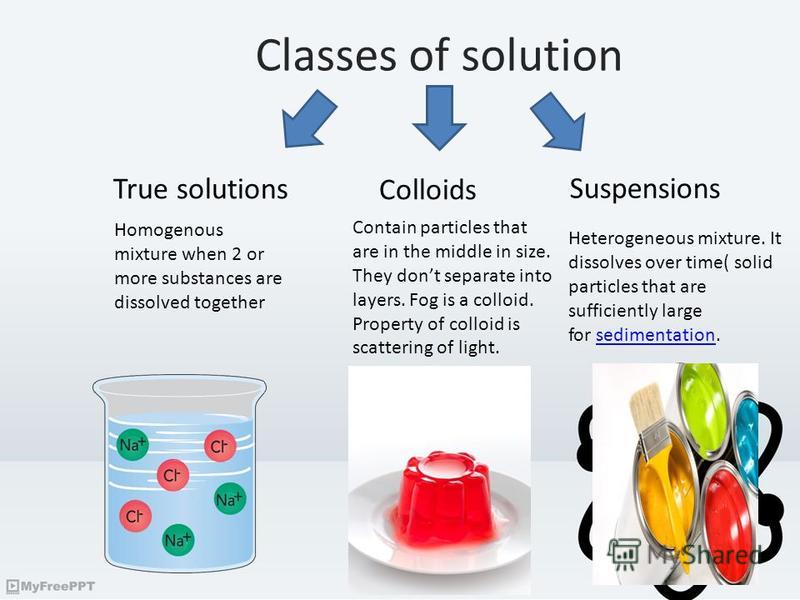
Suspension Solution Colloid Smoke . Compare colloids, suspensions, solutions, and find their differences. A suspension clears out, but a colloid doesn’t. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Understand particles in a solution, and explore solution, suspension, and colloid examples. When light passes through a colloidal. It is a quick and easy test that distinguishes a colloid or suspension from a solution. Not all colloids display the tyndall effect. Sometimes the dispersion medium is opaque. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Colloids are intermediaries between a suspension and a solution.
from www.myshared.ru
Understand particles in a solution, and explore solution, suspension, and colloid examples. Not all colloids display the tyndall effect. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Compare colloids, suspensions, solutions, and find their differences. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Sometimes the dispersion medium is opaque. A suspension clears out, but a colloid doesn’t. It is a quick and easy test that distinguishes a colloid or suspension from a solution. When light passes through a colloidal.
Презентация на тему "Colloidal systems. Classes of solution True
Suspension Solution Colloid Smoke Not all colloids display the tyndall effect. Understand particles in a solution, and explore solution, suspension, and colloid examples. Sometimes the dispersion medium is opaque. A suspension clears out, but a colloid doesn’t. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Compare colloids, suspensions, solutions, and find their differences. Not all colloids display the tyndall effect. When light passes through a colloidal. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Colloids are intermediaries between a suspension and a solution. It is a quick and easy test that distinguishes a colloid or suspension from a solution. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a.
From www.slideserve.com
PPT Colloids, Solutions, Suspensions PowerPoint Presentation, free Suspension Solution Colloid Smoke Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension clears out, but a colloid doesn’t. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Sometimes the dispersion medium is opaque. Understand particles in a solution, and explore solution, suspension, and colloid. Suspension Solution Colloid Smoke.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Suspension Solution Colloid Smoke It is a quick and easy test that distinguishes a colloid or suspension from a solution. Understand particles in a solution, and explore solution, suspension, and colloid examples. When light passes through a colloidal. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. Colloids are intermediaries between a suspension. Suspension Solution Colloid Smoke.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Suspension Solution Colloid Smoke A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Not all colloids display the tyndall effect. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Sometimes the dispersion medium is opaque. When light passes through a colloidal. Understand particles in. Suspension Solution Colloid Smoke.
From slideplayer.com
Suspensions and Colloids ppt download Suspension Solution Colloid Smoke A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. When light passes through a colloidal. Sometimes the dispersion medium is opaque. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Not all colloids display the tyndall effect. Suspensions and colloids. Suspension Solution Colloid Smoke.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation ID Suspension Solution Colloid Smoke Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Compare colloids, suspensions, solutions, and find their differences. Understand particles in a solution, and explore solution, suspension, and colloid examples. Sometimes the dispersion medium is opaque. When light passes through a colloidal. Suspensions and colloids are two common types of mixtures whose properties are. Suspension Solution Colloid Smoke.
From www.youtube.com
Science 6 Q1 Week 2 Solution, Suspension, Colloid YouTube Suspension Solution Colloid Smoke Understand particles in a solution, and explore solution, suspension, and colloid examples. A suspension clears out, but a colloid doesn’t. Colloids are intermediaries between a suspension and a solution. Sometimes the dispersion medium is opaque. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. Compare colloids, suspensions, solutions, and. Suspension Solution Colloid Smoke.
From www.showme.com
Solutions, Suspensions, and Colloids Science, Chemistry ShowMe Suspension Solution Colloid Smoke Not all colloids display the tyndall effect. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Understand particles in a solution, and explore solution, suspension, and colloid examples. Sometimes the dispersion medium is opaque. Compare colloids, suspensions, solutions, and find their differences. Suspensions and colloids are two common types of. Suspension Solution Colloid Smoke.
From slideplayer.com
Solutions Edward Wen. ppt download Suspension Solution Colloid Smoke Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. A suspension clears out, but a colloid doesn’t. Not all colloids display the tyndall effect. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Both suspensions and colloids are heterogeneous. Suspension Solution Colloid Smoke.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Suspension Solution Colloid Smoke When light passes through a colloidal. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. It is a quick and easy test that distinguishes a colloid or suspension from a solution. Not all colloids display the tyndall effect.. Suspension Solution Colloid Smoke.
From www.slideserve.com
PPT Emulsions & Colloids PowerPoint Presentation, free download ID Suspension Solution Colloid Smoke Sometimes the dispersion medium is opaque. Colloids are intermediaries between a suspension and a solution. A suspension clears out, but a colloid doesn’t. Understand particles in a solution, and explore solution, suspension, and colloid examples. Not all colloids display the tyndall effect. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a. Suspension Solution Colloid Smoke.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Solution Colloid Smoke Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. Compare colloids, suspensions, solutions, and find their differences. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. When light passes through a colloidal. It is a quick and easy test. Suspension Solution Colloid Smoke.
From www.youtube.com
Science 9 Chapter 2 Suspension, Colloid, Tyndall effect, Comparison of Suspension Solution Colloid Smoke When light passes through a colloidal. Not all colloids display the tyndall effect. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Compare colloids, suspensions, solutions, and find their differences. Colloids are intermediaries between a suspension and a solution. A suspension clears out, but a colloid doesn’t. Suspensions and colloids. Suspension Solution Colloid Smoke.
From www.youtube.com
SOLUTION, SUSPENSION AND COLLOID MIXTURES CHEMISTRY 720p YouTube Suspension Solution Colloid Smoke It is a quick and easy test that distinguishes a colloid or suspension from a solution. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. A suspension clears out, but a colloid doesn’t. Understand particles in a solution, and explore solution, suspension, and colloid examples. Not all colloids display. Suspension Solution Colloid Smoke.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension Solution Colloid Smoke Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Colloids are intermediaries between a suspension and a solution. When light passes through a colloidal. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. A suspension clears out, but a colloid doesn’t. It is. Suspension Solution Colloid Smoke.
From www.scribd.com
Solutions Suspensions and Colloids PDF Solution Colloid Suspension Solution Colloid Smoke Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. When light passes through a colloidal. Compare colloids, suspensions, solutions, and find their differences. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Sometimes the dispersion medium is opaque. Not all colloids display. Suspension Solution Colloid Smoke.
From www.youtube.com
What is solution, Suspension and Colloid? Science Composition of Suspension Solution Colloid Smoke Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. A suspension clears out, but a colloid doesn’t. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. It is a quick and easy test that distinguishes a colloid or suspension. Suspension Solution Colloid Smoke.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension Solution Colloid Smoke A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Not all colloids display the tyndall effect. When light passes through a colloidal. Colloids are intermediaries between a suspension and a solution. It is a quick and easy test that distinguishes a colloid or suspension from a solution. Compare colloids, suspensions,. Suspension Solution Colloid Smoke.
From www.youtube.com
Solution, Suspension & Colloid Science Experiment kit YouDo STEM Suspension Solution Colloid Smoke When light passes through a colloidal. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. A suspension clears out, but a colloid doesn’t. Colloids are intermediaries between a suspension and a solution. It is a quick and easy test that distinguishes a colloid or suspension from a solution. Understand. Suspension Solution Colloid Smoke.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Solution Colloid Smoke Not all colloids display the tyndall effect. Sometimes the dispersion medium is opaque. Compare colloids, suspensions, solutions, and find their differences. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Colloids are intermediaries between. Suspension Solution Colloid Smoke.
From slideplayer.com
A substance that cannot be broken down into simpler substances is ppt Suspension Solution Colloid Smoke Not all colloids display the tyndall effect. When light passes through a colloidal. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. A suspension clears out, but a colloid doesn’t. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Sometimes. Suspension Solution Colloid Smoke.
From www.thoughtco.com
Tyndall Effect Definition and Examples Suspension Solution Colloid Smoke Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloids are. Suspension Solution Colloid Smoke.
From www.slideserve.com
PPT Colloids, Solutions, Suspensions PowerPoint Presentation, free Suspension Solution Colloid Smoke When light passes through a colloidal. A suspension clears out, but a colloid doesn’t. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. It is a quick and easy test that. Suspension Solution Colloid Smoke.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Solution Colloid Smoke Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. Not all colloids display the tyndall effect. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Compare colloids, suspensions, solutions, and find their differences. Colloids are intermediaries between a suspension and a solution.. Suspension Solution Colloid Smoke.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension Solution Colloid Smoke Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. When light passes through a colloidal. Not all colloids display the tyndall effect. A suspension clears out, but a colloid doesn’t. It is a quick and easy test that distinguishes a colloid or suspension from a solution. Sometimes the dispersion medium is opaque. A. Suspension Solution Colloid Smoke.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Solution Colloid Smoke Sometimes the dispersion medium is opaque. Understand particles in a solution, and explore solution, suspension, and colloid examples. Not all colloids display the tyndall effect. Colloids are intermediaries between a suspension and a solution. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A suspension clears out, but a colloid. Suspension Solution Colloid Smoke.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID Suspension Solution Colloid Smoke A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A suspension clears out, but a colloid doesn’t. Not all colloids display the tyndall effect. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Both suspensions and colloids are heterogeneous mixtures,. Suspension Solution Colloid Smoke.
From slideplayer.com
Elements, Compounds, and Mixtures ppt download Suspension Solution Colloid Smoke Understand particles in a solution, and explore solution, suspension, and colloid examples. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Sometimes the dispersion medium is opaque. Compare colloids, suspensions, solutions, and find their differences. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those. Suspension Solution Colloid Smoke.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Suspension Solution Colloid Smoke Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension clears out, but a colloid doesn’t. Understand particles in a solution, and explore solution, suspension, and colloid examples. It is a quick and easy test that distinguishes a colloid or suspension from a solution. A colloid is a heterogeneous mixture whose particle. Suspension Solution Colloid Smoke.
From www.scribd.com
Classification and Properties of Solutions, Suspensions, and Colloids Suspension Solution Colloid Smoke Sometimes the dispersion medium is opaque. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloids are intermediaries between a suspension and a solution. A suspension clears out, but a colloid doesn’t. It is. Suspension Solution Colloid Smoke.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspension Solution Colloid Smoke A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Understand particles in a solution, and explore solution, suspension, and colloid examples. It is a quick and easy test that distinguishes a colloid or suspension from a solution. Not all colloids display the tyndall effect. Sometimes the dispersion medium is opaque.. Suspension Solution Colloid Smoke.
From ar.inspiredpencil.com
Colloids Suspensions And Solutions Suspension Solution Colloid Smoke Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. It is. Suspension Solution Colloid Smoke.
From www.youtube.com
Solution Suspension Colloid YouTube Suspension Solution Colloid Smoke A suspension clears out, but a colloid doesn’t. It is a quick and easy test that distinguishes a colloid or suspension from a solution. Not all colloids display the tyndall effect. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Colloids are intermediaries between a suspension and a solution. Both. Suspension Solution Colloid Smoke.
From www.youtube.com
Solution, Colloid or Suspension? The definition in Chemistry and Suspension Solution Colloid Smoke Sometimes the dispersion medium is opaque. When light passes through a colloidal. Not all colloids display the tyndall effect. A suspension clears out, but a colloid doesn’t. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. It is a quick and easy test that distinguishes a colloid or suspension from a solution. Colloids. Suspension Solution Colloid Smoke.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension Solution Colloid Smoke A suspension clears out, but a colloid doesn’t. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. When light passes through a colloidal. Sometimes the dispersion medium is opaque. Both. Suspension Solution Colloid Smoke.
From www.youtube.com
Module 13 Lesson 3 Suspensions and Colloids YouTube Suspension Solution Colloid Smoke A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A suspension clears out, but a colloid doesn’t. A colloid is a heterogeneous mixture whose particle size is intermediate between those of a solution and a suspension. Not all colloids display the tyndall effect. Colloids are intermediaries between a suspension and. Suspension Solution Colloid Smoke.