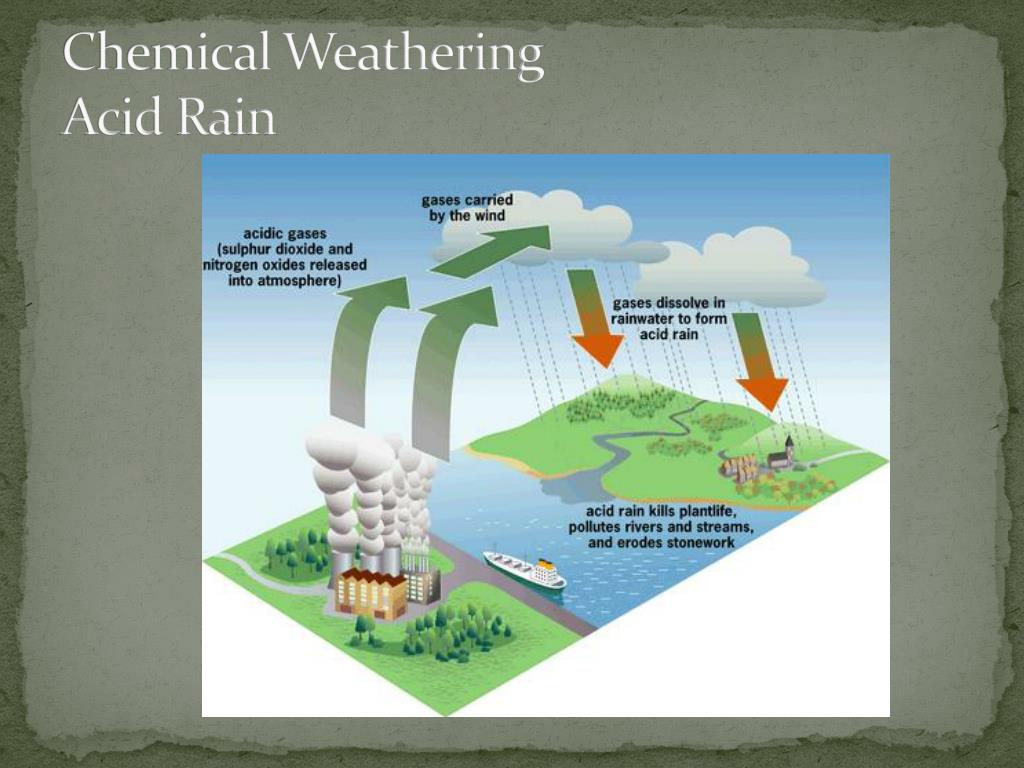
Acid Rain And Marble Chemical Reaction . Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid precipitation affects stone primarily in two ways: Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When atmospheric pollutants like oxides of nitrogen and sulphur.
from www.slideserve.com
When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid precipitation affects stone primarily in two ways: Marble and limestone both consist of calcium carbonate. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When atmospheric pollutants like oxides of nitrogen and sulphur. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt.
PPT Weathering PowerPoint Presentation, free download ID2135916
Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Acid precipitation affects stone primarily in two ways: When atmospheric pollutants like oxides of nitrogen and sulphur. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3.
From www.numerade.com
SOLVEDWrite reactions to show how the nitric and sulfuric acids in Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. Acid precipitation affects stone primarily in two ways: When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. When sulfurous, sulfuric, and nitric acids in polluted air and rain react. Acid Rain And Marble Chemical Reaction.
From www.slideserve.com
PPT Weathering PowerPoint Presentation, free download ID2135916 Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in. Acid Rain And Marble Chemical Reaction.
From ar.inspiredpencil.com
Acid Precipitation Acid Rain And Marble Chemical Reaction Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of. Acid Rain And Marble Chemical Reaction.
From brainly.in
Write the mechanism of acid rain? what is marble cancer? how is it Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. When sulfurous, sulfuric, and nitric acids in polluted air and rain react. Acid Rain And Marble Chemical Reaction.
From www.britannica.com
What Happened to Acid Rain? Britannica Acid Rain And Marble Chemical Reaction When atmospheric pollutants like oxides of nitrogen and sulphur. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). Acid precipitation affects stone primarily in. Acid Rain And Marble Chemical Reaction.
From cuprum.media
Кислотные дожди опасны? Купрум Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. Acid precipitation affects stone primarily in two ways: Marble and limestone both consist of calcium carbonate. Acid rain, as the name suggests, can be said. Acid Rain And Marble Chemical Reaction.
From www.vecteezy.com
Acid rain, as sulfuric or nitric acid that fall to the ground from the Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate. Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the. Acid Rain And Marble Chemical Reaction.
From blog.stuidapp.com
Acid rain Blog Stuid Learning App Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid precipitation affects stone primarily in two ways: Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When atmospheric pollutants like oxides of nitrogen and sulphur. Marble and. Acid Rain And Marble Chemical Reaction.
From chemistry-chemists.com
Качественные реакции. Qualitative reactions Химия и Химики № 4 2021 Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When atmospheric pollutants like oxides of nitrogen and sulphur. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Acid precipitation affects stone primarily in two ways: Marble and limestone both consist of calcium carbonate.. Acid Rain And Marble Chemical Reaction.
From socratic.org
How does acid rain form? Socratic Acid Rain And Marble Chemical Reaction When atmospheric pollutants like oxides of nitrogen and sulphur. Acid precipitation affects stone primarily in two ways: When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). Acid rain,. Acid Rain And Marble Chemical Reaction.
From www.youtube.com
Marble Reacts with Acid YouTube Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble. Acid Rain And Marble Chemical Reaction.
From webmis.highland.cc.il.us
The Chemistry of Acid Rain Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Acid rain, as the name suggests, can. Acid Rain And Marble Chemical Reaction.
From mstimms-gcse.blogspot.com
Ms Timms GCSE Limestone reaction cycle chemistry Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid precipitation affects stone primarily in two ways: When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite. Acid Rain And Marble Chemical Reaction.
From slideplayer.com
CWK CWK Acid Rain State the adverse effect of these common pollutants Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Marble and limestone both consist of calcium carbonate. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone,. Acid Rain And Marble Chemical Reaction.
From brainly.in
what is meant by acid rain Brainly.in Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. When atmospheric pollutants like oxides of nitrogen and sulphur. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). Acid rain,. Acid Rain And Marble Chemical Reaction.
From ian.umces.edu
Causes of acid rain Media Library Integration and Application Network Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). When sulfurous,. Acid Rain And Marble Chemical Reaction.
From softbacktravel.com
What is Acid Rain & How is it Formed? Softback Travel Acid Rain And Marble Chemical Reaction Acid precipitation affects stone primarily in two ways: Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate. When atmospheric pollutants like oxides of nitrogen and sulphur. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid. Acid Rain And Marble Chemical Reaction.
From fphoto.photoshelter.com
acid rain environment marble atmospheric pollution Fundamental Acid Rain And Marble Chemical Reaction Acid precipitation affects stone primarily in two ways: When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Acid Rain And Marble Chemical Reaction.
From www.slideserve.com
PPT WEATHERING PowerPoint Presentation ID314244 Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). Acid precipitation affects. Acid Rain And Marble Chemical Reaction.
From www.javatpoint.com
Acid Rain Definition JavaTpoint Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. When atmospheric pollutants like oxides of nitrogen and sulphur. Acid precipitation affects stone primarily in two. Acid Rain And Marble Chemical Reaction.
From brainly.in
a solution reacts with marble chips to produce a gas which turns lime Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the. Acid Rain And Marble Chemical Reaction.
From pollutantsinthegreatlakes.weebly.com
Acid Rain pollution in the great lakes Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. When atmospheric pollutants like oxides of nitrogen. Acid Rain And Marble Chemical Reaction.
From www.slideserve.com
PPT Chapter 19 “Acids, Bases, and Salts” PowerPoint Presentation ID Acid Rain And Marble Chemical Reaction Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h. Acid Rain And Marble Chemical Reaction.
From www.youtube.com
Chemical reaction of marble to acid YouTube Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate. Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in. Acid Rain And Marble Chemical Reaction.
From hhsvoyager.org
How does acid rain affect the ecosystem? The Voyager Acid Rain And Marble Chemical Reaction Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the weak acid h 2 co 3. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and. Acid Rain And Marble Chemical Reaction.
From www.slideserve.com
PPT Chemical Weathering of Rocks PowerPoint Presentation, free Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium. Acid Rain And Marble Chemical Reaction.
From ar.inspiredpencil.com
Chemical Weathering Acid Rain Statue Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt derived from the. Acid Rain And Marble Chemical Reaction.
From www.tes.com
Limestone Erosion and Acid Rain Teaching Resources Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Acid precipitation affects stone primarily in two ways: This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Acid rain, as. Acid Rain And Marble Chemical Reaction.
From www.teachoo.com
Acid Rain Definition, Causes, Effects Teachoo Concepts Acid Rain And Marble Chemical Reaction When atmospheric pollutants like oxides of nitrogen and sulphur. Acid rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. Acid precipitation affects stone primarily in two ways: When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and. Acid Rain And Marble Chemical Reaction.
From www.sliderbase.com
AcidBase Reactions Presentation Chemistry Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). Acid precipitation affects stone primarily in two ways: When atmospheric pollutants like oxides of nitrogen. Acid Rain And Marble Chemical Reaction.
From www.slideserve.com
PPT AcidBase Theories PowerPoint Presentation, free download ID Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. When atmospheric pollutants like oxides of nitrogen and sulphur. Acid rain, as the name suggests, can. Acid Rain And Marble Chemical Reaction.
From rogerjcheng.com
ACID RAIN'S EFFECT on MARBLE STRUCTURESANALYTICAL CHEMISTRY Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Acid rain, as the name suggests, can be said as the precipitation of acid in the form. Acid Rain And Marble Chemical Reaction.
From www.youtube.com
Acid Rain Equations YouTube Acid Rain And Marble Chemical Reaction When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. Marble and limestone both consist of calcium. Acid Rain And Marble Chemical Reaction.
From www.worldatlas.com
What Is Acid Rain? WorldAtlas Acid Rain And Marble Chemical Reaction This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. When atmospheric pollutants like oxides of nitrogen and sulphur. Marble and limestone both consist of calcium carbonate. Acid rain,. Acid Rain And Marble Chemical Reaction.
From www.slideserve.com
PPT Weathering & Erosion PowerPoint Presentation, free download ID Acid Rain And Marble Chemical Reaction Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. This video shows how carrara marble reacts to acid (at 1.0 n, which is ~10000 times more aggressive than the worst acid rain!). Marble and limestone both consist of calcium carbonate (caco 3 ), a salt. When sulfurous, sulfuric, and nitric acids in polluted air react with. Acid Rain And Marble Chemical Reaction.