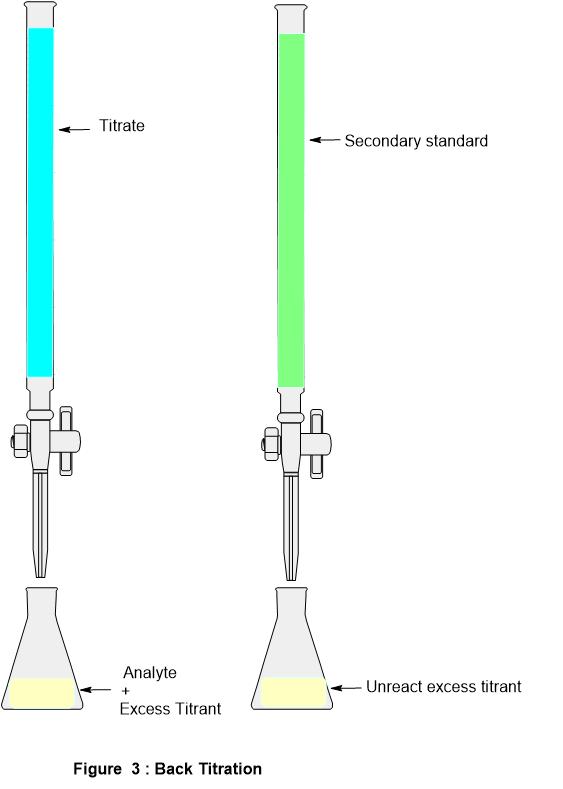
Titration Assumptions . a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. in a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
from www.chemistryscl.com
learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. in a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it.
Titrimetry, Titration Classifications, Standard solutions, Equivalence
Titration Assumptions a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. in a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the.
From docslib.org
Titrations in Analytical Chemistry Titration Methods Are Based On DocsLib Titration Assumptions The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. a titration is the process of determining the quantity of one substance by adding. Titration Assumptions.
From relationshipbetween.com
Difference Between Titration And Neutralization Relationship Between Titration Assumptions titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. a titration is the process of determining. Titration Assumptions.
From www.youtube.com
Back Titration Example YouTube Titration Assumptions a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is the process of determining the quantity of. Titration Assumptions.
From www.mdpi.com
Healthcare Free FullText Factors Associated with the Titration Assumptions learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. At the equivalence point in a neutralization, the moles of acid are equal to the moles of. Titration Assumptions.
From chem-textbook.ucalgary.ca
Finding the Endpoint of a Titration UCalgary Chemistry Textbook Titration Assumptions titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is the process of determining the quantity of a substance a by. Titration Assumptions.
From www.tes.com
KS3 GCSE Chemistry Introduction To Titrations Lesson Presentation and Titration Assumptions a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is the process of determining the quantity of one substance by adding measured. Titration Assumptions.
From chem-textbook.ucalgary.ca
Finding the Endpoint of a Titration UCalgary Chemistry Textbook Titration Assumptions The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. a titration is the process of determining the. Titration Assumptions.
From lessonplans.ie
Titrations list Lesson Plans Titration Assumptions At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The. Titration Assumptions.
From studylib.net
EDTA Titrations Analytical Chemistry Titration Assumptions a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. The basic process involves adding a standard solution of one reagent to a known. Titration Assumptions.
From www.pinterest.com.au
titration process and its application Chemistry practical, Teaching Titration Assumptions titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. a titration is a laboratory technique used. Titration Assumptions.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration Assumptions a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. Titration Assumptions.
From chemistnotes.com
Karl Fischer Titration easy principle, 2 types, advantages Titration Assumptions The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. in a ph titration you measure the ph as a function of the volume of. Titration Assumptions.
From www.researchgate.net
ADP titrations either in the presence (AC) or absence (DF) of Titration Assumptions a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. titration involves the gradual addition of a reagent of. Titration Assumptions.
From www.numerade.com
SOLVED To sketch the titration curve for a weak acid we approximate Titration Assumptions titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. a titration is a laboratory technique used to precisely measure molar concentration of. Titration Assumptions.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Titration Assumptions a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. titration involves the gradual addition of a reagent of known. Titration Assumptions.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Assumptions The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. learn about the titration technique through a practical experiment to determine the reacting volumes. Titration Assumptions.
From www.tandfonline.com
Automated system for performing pHbased titrations Instrumentation Titration Assumptions in a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is the process of determining the quantity of a. Titration Assumptions.
From www.odinity.com
Titration of a Diprotic Acid Identifying and Unknown Technical Memo Titration Assumptions a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is the process of determining the quantity of a substance a by adding measured increments of. Titration Assumptions.
From www.cphi-online.com
KBI Biopharma Inc. Titration Curve Modeling in Bioassay Potency Method Titration Assumptions titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. in a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the. At the equivalence point in a neutralization, the moles of acid. Titration Assumptions.
From authentictigershop.com
Understanding the Equivalence Point in Titrations Authentic News Daily Titration Assumptions At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. . Titration Assumptions.
From msumgenchem.blogspot.com
Dr. Bodwin's General Chemistry Blog Titrations I Monoprotic/Monoprotic Titration Assumptions The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. learn about the titration technique through a practical experiment to determine the reacting volumes. Titration Assumptions.
From docworksheet.com
Gcse Titration Calculations Worksheet Titration Assumptions learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. in a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the. a titration is the process of determining the quantity of a substance. Titration Assumptions.
From www.slideserve.com
PPT RABI RASHIDI UNIVERSITY CHEMISTRY LAB TITRATION PowerPoint Titration Assumptions learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. The basic process involves adding a standard solution of one reagent to a known amount of the unknown. Titration Assumptions.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Assumptions titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. a titration is a laboratory technique used to precisely measure molar concentration of. Titration Assumptions.
From slideplayer.com
Chemistry NCEA L3 3.6 Aqueous Systems ppt download Titration Assumptions At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. learn about the titration technique through a practical experiment to determine the reacting. Titration Assumptions.
From vasadylanpeake.blogspot.com
Back Titration Method Dylan Peake Titration Assumptions At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. learn about the titration technique through a practical experiment to determine the reacting. Titration Assumptions.
From www.youtube.com
Titrations Analytical Techniques Me Study YouTube Titration Assumptions a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. Titration Assumptions.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Assumptions in a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. a titration is the process of determining the quantity of one substance. Titration Assumptions.
From www.youtube.com
Errors in titrations YouTube Titration Assumptions in a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. The basic process involves adding a standard solution of one reagent. Titration Assumptions.
From www.studocu.com
Chapter 14 Acid Base Titration Calculations in the context of Titration Assumptions a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of. Titration Assumptions.
From studylib.net
EXPERIMENT 3. ACIDBASE TITRATIONS DETERMINATION OF Titration Assumptions a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. a titration is defined as ‘the process of determining the quantity of a substance a. Titration Assumptions.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Assumptions The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. a titration is defined as ‘the process of determining the quantity of a substance a by. Titration Assumptions.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation ID6602548 Titration Assumptions learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. a titration is defined as ‘the process of determining the quantity of a substance a by adding measured increments of substance b, the titrant, with which it. titration involves the gradual addition of a reagent of. Titration Assumptions.
From www.youtube.com
Back titration YouTube Titration Assumptions a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. a titration is the process of determining the quantity of a substance a by adding measured increments of substance b ,. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.. Titration Assumptions.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Assumptions learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. The basic process involves adding a standard solution of one reagent to a known amount of the unknown. Titration Assumptions.