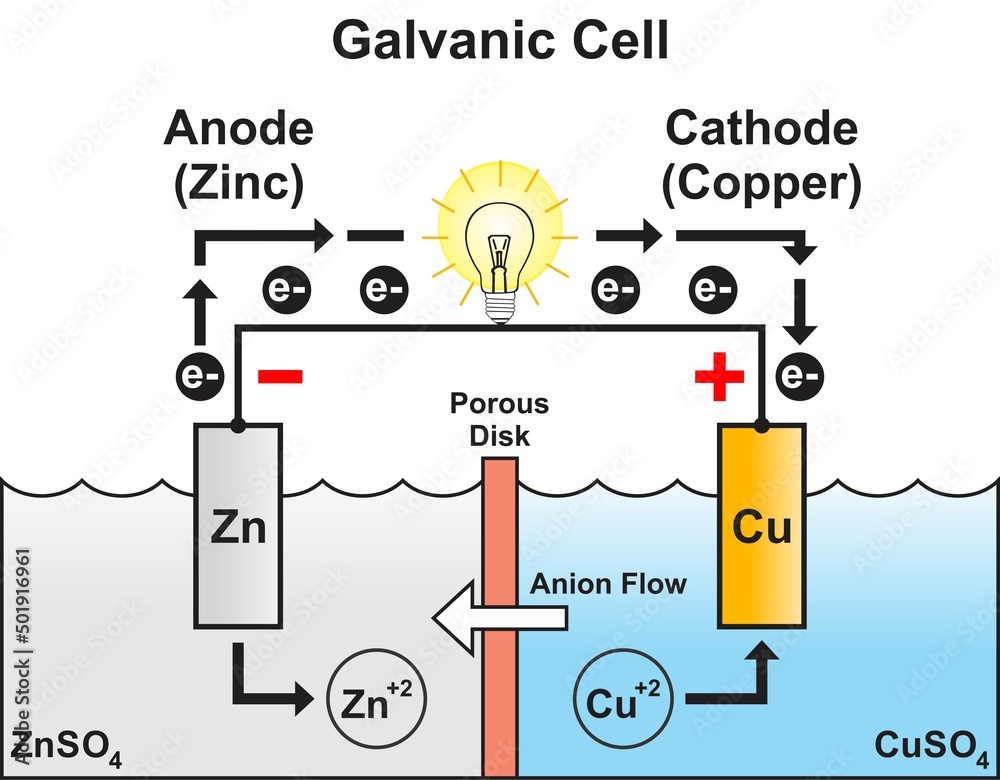
Zinc And Copper Cell Diagram . revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: a zinc sulfate solution is floated on top of the copper sulfate solution; the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The reactions occurring are those. Then a zinc electrode is placed in the zinc sulfate solution. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions.
from stock.adobe.com
in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). The reactions occurring are those. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. a zinc sulfate solution is floated on top of the copper sulfate solution; zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. Then a zinc electrode is placed in the zinc sulfate solution.
Galvanic voltaic cell infographic diagram battery part structure
Zinc And Copper Cell Diagram galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. Then a zinc electrode is placed in the zinc sulfate solution. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. a zinc sulfate solution is floated on top of the copper sulfate solution; the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The reactions occurring are those.
From www.iswori.com.np
Electrochemistry Class 12 Chemistry Notes NEB Notes Zinc And Copper Cell Diagram when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). revision notes on 5.4.1 representing cells for the aqa a. Zinc And Copper Cell Diagram.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Zinc And Copper Cell Diagram the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. galvanic cell with zinc and copper depicts a voltaic cell and. Zinc And Copper Cell Diagram.
From exowsidwq.blob.core.windows.net
Zinc How To Find Charge at Patel blog Zinc And Copper Cell Diagram galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. The reactions occurring are those. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: revision notes on 5.4.1 representing cells for the aqa. Zinc And Copper Cell Diagram.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID5368420 Zinc And Copper Cell Diagram galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. Then a zinc electrode is placed in the zinc sulfate solution. a zinc sulfate solution is floated on top of the copper sulfate solution; revision notes on 5.4.1 representing cells for the aqa a level chemistry. Zinc And Copper Cell Diagram.
From www.researchgate.net
(PDF) Better Batteries Through Electrochemistry Zinc And Copper Cell Diagram Then a zinc electrode is placed in the zinc sulfate solution. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4).. Zinc And Copper Cell Diagram.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Zinc And Copper Cell Diagram galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. The reactions occurring are those. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: zinc behaves as the anode (supplying electrons) of the. Zinc And Copper Cell Diagram.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Zinc And Copper Cell Diagram Then a zinc electrode is placed in the zinc sulfate solution. The reactions occurring are those. the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate,. Zinc And Copper Cell Diagram.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Zinc And Copper Cell Diagram in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. a zinc sulfate solution is floated on top of the copper sulfate solution; revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. . Zinc And Copper Cell Diagram.
From www.shutterstock.com
Diagrama de células electroquímicas. Célula galvánica vector de stock Zinc And Copper Cell Diagram the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). when a zinc rod is inserted into a. Zinc And Copper Cell Diagram.
From wps.prenhall.com
Media Portfolio Zinc And Copper Cell Diagram in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). revision notes on 5.4.1 representing cells for the aqa a level. Zinc And Copper Cell Diagram.
From colouremployer8.gitlab.io
Marvelous Copper Zinc Battery Reaction Cie Chemistry A Level Syllabus Zinc And Copper Cell Diagram zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). Then a zinc electrode is placed in the zinc sulfate solution. a zinc sulfate solution is floated on top of the copper sulfate solution; galvanic cell with zinc and copper depicts a voltaic cell and shows how the. Zinc And Copper Cell Diagram.
From www.scienceabc.com
(Photo Credit Nandalal Sarkar/Shutterstock) Zinc And Copper Cell Diagram Then a zinc electrode is placed in the zinc sulfate solution. revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4).. Zinc And Copper Cell Diagram.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Zinc And Copper Cell Diagram galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: The reactions occurring are those. Then a zinc electrode is placed in the zinc sulfate. Zinc And Copper Cell Diagram.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Zinc And Copper Cell Diagram zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. a zinc sulfate solution is floated on top of the copper sulfate solution; revision notes on. Zinc And Copper Cell Diagram.
From www.vrogue.co
Science Clipart And Diagrams Galvanic Cell Science Cl vrogue.co Zinc And Copper Cell Diagram the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). Then a zinc electrode is placed in the zinc sulfate solution. galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. . Zinc And Copper Cell Diagram.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER Zinc And Copper Cell Diagram zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). a zinc sulfate solution is floated on top of the copper sulfate solution; revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The reactions occurring. Zinc And Copper Cell Diagram.
From courses.lumenlearning.com
Galvanic Cells Chemistry Zinc And Copper Cell Diagram zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). galvanic cell with zinc and copper depicts a voltaic cell and shows how. Zinc And Copper Cell Diagram.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General Zinc And Copper Cell Diagram The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). in a spontaneous reaction electrons leave the zinc,. Zinc And Copper Cell Diagram.
From brainly.in
label the parts of simple electrical cell (diagram given above Zinc And Copper Cell Diagram when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). a zinc sulfate solution is floated on top of the copper. Zinc And Copper Cell Diagram.
From saylordotorg.github.io
Electrochemistry Zinc And Copper Cell Diagram Then a zinc electrode is placed in the zinc sulfate solution. The reactions occurring are those. the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction. Zinc And Copper Cell Diagram.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Zinc And Copper Cell Diagram a zinc sulfate solution is floated on top of the copper sulfate solution; in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. The reactions occurring are those. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a. Zinc And Copper Cell Diagram.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Zinc And Copper Cell Diagram The reactions occurring are those. revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. zinc behaves as the anode (supplying electrons) of the. Zinc And Copper Cell Diagram.
From www.coursehero.com
[Solved] Sketch a diagram of a copper/zinc Daniell cell. Label all the Zinc And Copper Cell Diagram revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. a zinc sulfate solution is floated on top of the copper sulfate solution; . Zinc And Copper Cell Diagram.
From integrated-mcat.com
Science Image Archive for Teachers Zinc And Copper Cell Diagram galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. a zinc sulfate solution is floated on top of the copper sulfate solution; revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The. Zinc And Copper Cell Diagram.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Zinc And Copper Cell Diagram a zinc sulfate solution is floated on top of the copper sulfate solution; zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Then a zinc. Zinc And Copper Cell Diagram.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Zinc And Copper Cell Diagram galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. The reactions occurring are those. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. a zinc sulfate solution is floated on top of the. Zinc And Copper Cell Diagram.
From www.embibe.com
How can you electroplate an iron nail with copper Explain with the help Zinc And Copper Cell Diagram in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. Then a zinc electrode is placed in the zinc sulfate solution. The reactions occurring are those. galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric.. Zinc And Copper Cell Diagram.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Zinc And Copper Cell Diagram revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. a zinc sulfate solution is floated on top of the copper sulfate solution; the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper. Zinc And Copper Cell Diagram.
From quizlet.com
Label the zinc/copper galvanic cell Diagram Quizlet Zinc And Copper Cell Diagram revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: zinc behaves as the anode (supplying electrons) of the galvanic cell and the. Zinc And Copper Cell Diagram.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2810901 Zinc And Copper Cell Diagram the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: zinc behaves as the anode (supplying electrons) of the galvanic. Zinc And Copper Cell Diagram.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Zinc And Copper Cell Diagram in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. The reactions occurring are those. Then a zinc electrode is placed in the zinc sulfate solution. the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains. Zinc And Copper Cell Diagram.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper Zinc And Copper Cell Diagram the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). revision notes on 5.4.1 representing cells for the aqa a level chemistry syllabus,. Zinc And Copper Cell Diagram.
From classnotes.org.in
Extraction of Copper and Zinc Chemistry, Class 12, General Principles Zinc And Copper Cell Diagram galvanic cell with zinc and copper depicts a voltaic cell and shows how the oxidation and reduction reactions yield an electric. The reactions occurring are those. the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). revision notes on 5.4.1 representing cells. Zinc And Copper Cell Diagram.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID1195562 Zinc And Copper Cell Diagram when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: a zinc sulfate solution is floated on top of the copper sulfate solution; zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). the first. Zinc And Copper Cell Diagram.
From courses.lumenlearning.com
Galvanic Cells Chemistry Zinc And Copper Cell Diagram a zinc sulfate solution is floated on top of the copper sulfate solution; the first beaker contains zinc sulfate (znso 4) into which a strip of zinc is dipped, while the adjacent beaker contains copper sulfate (cuso 4). when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous. Zinc And Copper Cell Diagram.