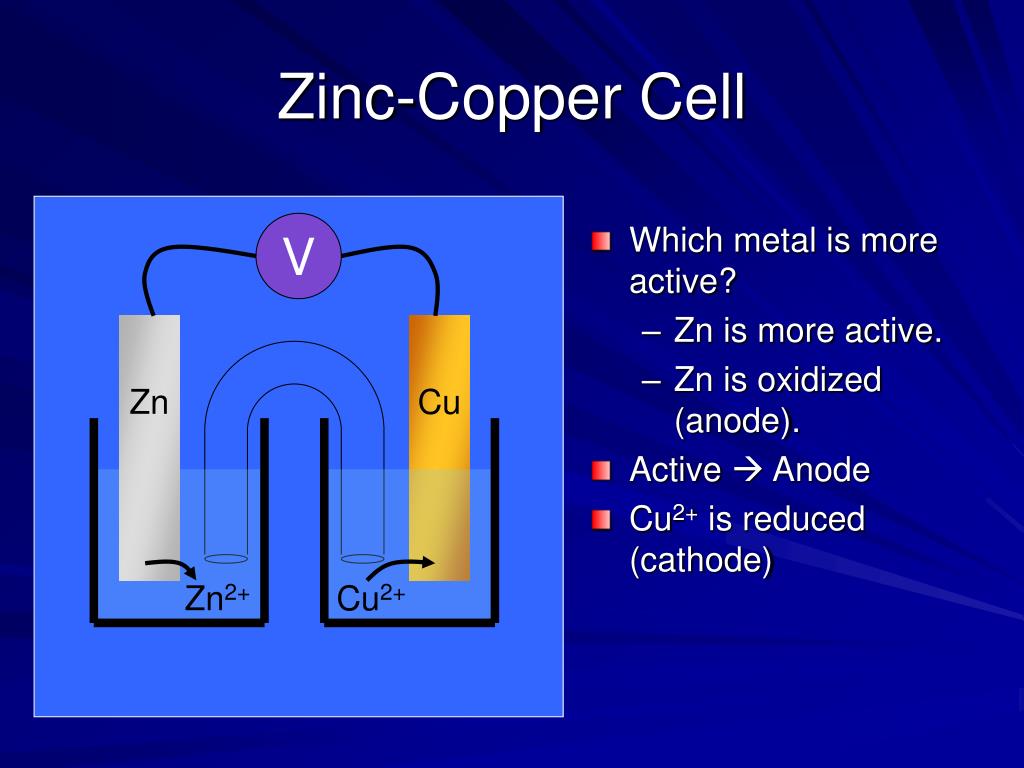
Copper And Zinc Gives . Connecting the copper electrode to the zinc electrode allows an electric current to flow. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. Zinc loses two of its. By observing this reaction and its products, and noting the difference in. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper.
from www.slideserve.com
This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. Zinc loses two of its. Connecting the copper electrode to the zinc electrode allows an electric current to flow. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. By observing this reaction and its products, and noting the difference in.
PPT Electrochemical Cells PowerPoint Presentation, free download ID
Copper And Zinc Gives This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. Zinc loses two of its. After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Connecting the copper electrode to the zinc electrode allows an electric current to flow. By observing this reaction and its products, and noting the difference in. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper.
From blog.thepipingmart.com
A Breakdown of Copper vs. Zinc Reactivity Copper And Zinc Gives By observing this reaction and its products, and noting the difference in. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Zinc loses two of its. After the copper adheres to the zinc metal, it can't dissolve anymore because if. Copper And Zinc Gives.
From www.slideserve.com
PPT Copper and zinc battery PowerPoint Presentation, free download Copper And Zinc Gives In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Connecting the copper electrode to the zinc electrode allows an electric current to flow. Zinc loses two of its. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. In the zn/cu. Copper And Zinc Gives.
From blog.thepipingmart.com
Advantages and Disadvantages of Copper Zinc Alloy Copper And Zinc Gives This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. Connecting the copper electrode to the zinc electrode allows an electric current to flow. Zinc loses two of its. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the. Copper And Zinc Gives.
From optimisingnutrition.com
Copper Rich Foods and Recipes A Practical Guide Optimising Nutrition Copper And Zinc Gives In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Connecting the copper electrode to the zinc electrode allows an electric current to flow. By observing this reaction and its products, and noting the difference in. Zinc loses two of its.. Copper And Zinc Gives.
From blog.thepipingmart.com
Zinc vs. Copper What's the Difference Copper And Zinc Gives Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. In this demonstration, dissolved copper ions come in contact with zinc, and zinc. Copper And Zinc Gives.
From www.youtube.com
2. The ratio of copper and zinc in brass is 13 7. How much zinc will Copper And Zinc Gives This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Copper (ii) oxide and zinc metal react together in. Copper And Zinc Gives.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6754031 Copper And Zinc Gives Zinc loses two of its. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Connecting the copper electrode to the zinc electrode allows an electric current to flow. This is an. Copper And Zinc Gives.
From blog.thepipingmart.com
CopperZinc Alloys An Overview Copper And Zinc Gives This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper.. Copper And Zinc Gives.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Copper And Zinc Gives This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Copper (ii) oxide and zinc metal react together in. Copper And Zinc Gives.
From www.youtube.com
An alloy of copper and zinc contains 45 copper; the rest is zinc. Find Copper And Zinc Gives Zinc loses two of its. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. By observing this reaction and its products, and noting the difference in. In this demonstration,. Copper And Zinc Gives.
From drjockers.tumblr.com
Dr Jockers — Copper and zinc are essential nutrients necessary... Copper And Zinc Gives This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does. Copper And Zinc Gives.
From www.thefoodstatecompany.com
Natural Zinc & Copper Supplement The Foodstate Company Copper And Zinc Gives After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Copper (ii) oxide and zinc metal react. Copper And Zinc Gives.
From www.thorne.com
Zinc Picolinate and Copper Supplements Thorne Copper And Zinc Gives This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce. Copper And Zinc Gives.
From foodsforhungry.blogspot.com
Foods With Zinc And Copper Foods Details Copper And Zinc Gives In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. Zinc loses two of its. By observing. Copper And Zinc Gives.
From chrismasterjohnphd.substack.com
030 How to manage the zinctocopper ratio and what to do if zinc and Copper And Zinc Gives Connecting the copper electrode to the zinc electrode allows an electric current to flow. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. By observing this reaction and its products, and noting the difference in. Zinc loses two of its. After the copper adheres to the zinc metal, it can't dissolve. Copper And Zinc Gives.
From www.researchgate.net
4 Hardness of Copper Zinc alloy and copper zinc alloy with 3 wt. and Copper And Zinc Gives In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Connecting the copper electrode to the zinc electrode allows an electric current to flow. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. Copper (ii) oxide and. Copper And Zinc Gives.
From blog.thepipingmart.com
CopperZinc Alloys A Complete Guide Copper And Zinc Gives Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal. Copper And Zinc Gives.
From blog.thepipingmart.com
An Introduction to CopperZinc Alloys Copper And Zinc Gives Connecting the copper electrode to the zinc electrode allows an electric current to flow. After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. In the zn/cu system,. Copper And Zinc Gives.
From blog.thepipingmart.com
An Overview of Separating Copper and Zinc Alloys Copper And Zinc Gives Connecting the copper electrode to the zinc electrode allows an electric current to flow. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the. Copper And Zinc Gives.
From express.adobe.com
Zinc and Copper Chloride Copper And Zinc Gives In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. Zinc loses two of its. Connecting the. Copper And Zinc Gives.
From www.slideserve.com
PPT Copper and zinc battery PowerPoint Presentation, free download Copper And Zinc Gives In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence. Copper And Zinc Gives.
From blog.thepipingmart.com
Difference Between Copper and Zinc Copper And Zinc Gives Zinc loses two of its. By observing this reaction and its products, and noting the difference in. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper. Copper And Zinc Gives.
From www.slideserve.com
PPT Electrochemistry I PowerPoint Presentation, free download ID Copper And Zinc Gives In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Connecting the copper electrode to the zinc electrode allows an electric. Copper And Zinc Gives.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Copper And Zinc Gives After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Copper (ii) oxide and zinc metal react. Copper And Zinc Gives.
From blog.thepipingmart.com
Copper Zinc Alloys Properties and Uses Copper And Zinc Gives In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. By observing this reaction and its products, and noting the difference in. In. Copper And Zinc Gives.
From fphoto.photoshelter.com
science chemistry redox reaction copper zinc Fundamental Photographs Copper And Zinc Gives After the copper adheres to the zinc metal, it can't dissolve anymore because if it, by chance, does dissolve, the zinc metal around it. Connecting the copper electrode to the zinc electrode allows an electric current to flow. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. This is an example. Copper And Zinc Gives.
From www.youtube.com
mixture of copper and zinc YouTube Copper And Zinc Gives In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. Connecting the copper electrode to the zinc electrode allows an electric current to flow. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and. Copper And Zinc Gives.
From blog.thepipingmart.com
Separating Copper and Zinc Alloys A StepbyStep Guide Copper And Zinc Gives In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper.. Copper And Zinc Gives.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Copper And Zinc Gives In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. By observing this reaction and its products, and noting the difference in. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. In the zn/cu system, the valence. Copper And Zinc Gives.
From foodsforhungry.blogspot.com
Foods With Zinc And Copper Foods Details Copper And Zinc Gives In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. By observing this reaction and its products, and noting the difference in. Connecting the copper electrode to the zinc electrode allows an electric current to flow. This is an example of. Copper And Zinc Gives.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Copper And Zinc Gives Zinc loses two of its. Connecting the copper electrode to the zinc electrode allows an electric current to flow. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. By observing this reaction and its products, and noting the difference in. This is an example of a cell without a. Copper And Zinc Gives.
From www.nfpt.com
Zinc and Copper Depletion What You Need to Know Copper And Zinc Gives Zinc loses two of its. In the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in copper because of shielding of the s electrons of. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. This is an example of a. Copper And Zinc Gives.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Copper And Zinc Gives Zinc loses two of its. By observing this reaction and its products, and noting the difference in. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. This is an example of. Copper And Zinc Gives.
From blog.thepipingmart.com
Comparing the Reactivity of Copper vs Zinc Copper And Zinc Gives In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. By observing this reaction and its products, and noting the difference in. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. Connecting the copper electrode to the. Copper And Zinc Gives.
From www.dreamstime.com
Copper and Zinc on the Periodic Table of the Elements on Black Copper And Zinc Gives Copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Zinc loses two of its. This is an example of a cell without a salt bridge, and ions may flow across the interface between the two solutions. Connecting the copper electrode to the zinc electrode allows an electric current to flow. After. Copper And Zinc Gives.