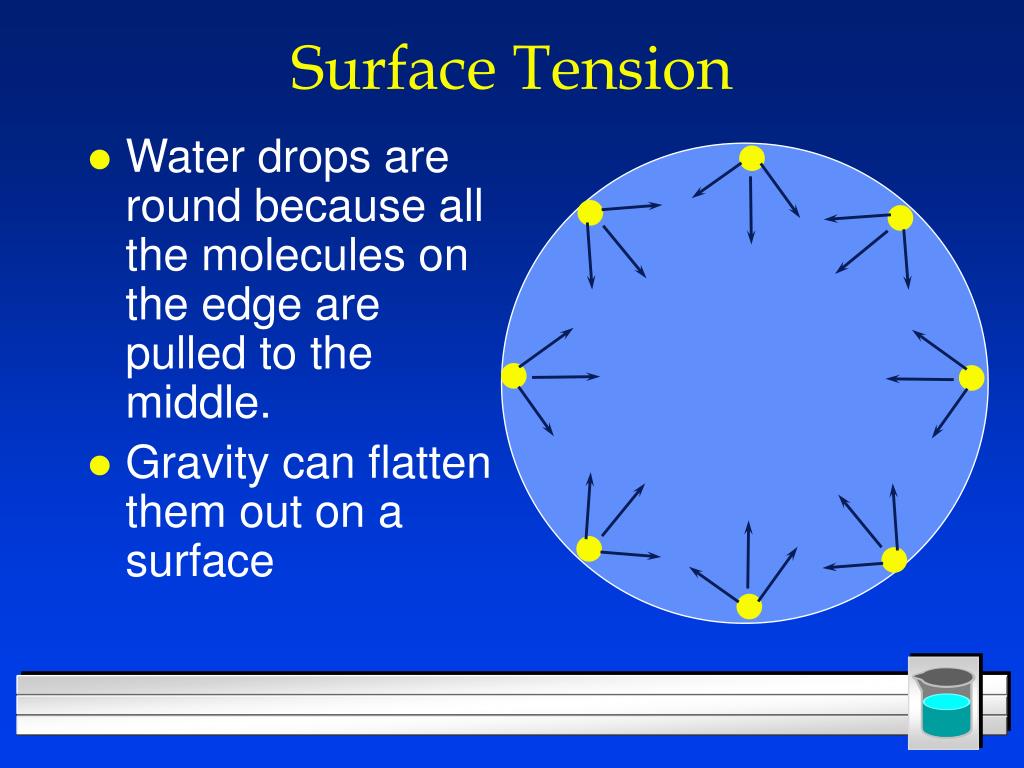
Which Has Higher Surface Tension Water Or Oil . If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water, soapy water, or rubbing alcohol. Since these intermolecular forces vary depending on the nature of the. In order to do this, you will determine the number of droplets In this portion of the lab you will determine which liquid has the highest surface tension: Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a high surface tension (72 dynes/cm).
from www.slideserve.com
Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water has a high surface tension (72 dynes/cm). Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In order to do this, you will determine the number of droplets If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Water, soapy water, or rubbing alcohol. Since these intermolecular forces vary depending on the nature of the. In this portion of the lab you will determine which liquid has the highest surface tension:
PPT Chapter 15 PowerPoint Presentation, free download ID245553
Which Has Higher Surface Tension Water Or Oil Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water, soapy water, or rubbing alcohol. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. In order to do this, you will determine the number of droplets If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. In this portion of the lab you will determine which liquid has the highest surface tension: Water has a high surface tension (72 dynes/cm).
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension Which Has Higher Surface Tension Water Or Oil Water has a high surface tension (72 dynes/cm). If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Water, soapy water, or rubbing alcohol. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Which Has Higher Surface Tension Water Or Oil.
From www.biolinscientific.com
Surface tension of water Why is it so high? Which Has Higher Surface Tension Water Or Oil Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water, soapy water, or rubbing alcohol. In this portion of the lab you will determine which liquid has the highest surface tension: In order to do this, you will determine the number of droplets Liquids with high surface tension exhibit. Which Has Higher Surface Tension Water Or Oil.
From www.sciencephoto.com
Surface tension of water Stock Image C038/9378 Science Photo Library Which Has Higher Surface Tension Water Or Oil In this portion of the lab you will determine which liquid has the highest surface tension: Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid. Which Has Higher Surface Tension Water Or Oil.
From fphoto.photoshelter.com
science chemistry surface tension Fundamental Photographs The Art Which Has Higher Surface Tension Water Or Oil In this portion of the lab you will determine which liquid has the highest surface tension: If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Surface tension is the energy, or work, required to increase the surface. Which Has Higher Surface Tension Water Or Oil.
From comsol.com
Tears of Wine and the Marangoni Effect COMSOL Blog Which Has Higher Surface Tension Water Or Oil If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the. Which Has Higher Surface Tension Water Or Oil.
From www.animalia-life.club
Surface Tension Of Water On A Leaf Which Has Higher Surface Tension Water Or Oil Water has a high surface tension (72 dynes/cm). Since these intermolecular forces vary depending on the nature of the. In this portion of the lab you will determine which liquid has the highest surface tension: In order to do this, you will determine the number of droplets Surface tension is the energy, or work, required to increase the surface area. Which Has Higher Surface Tension Water Or Oil.
From errantscience.com
Water surface tension ErrantScience Which Has Higher Surface Tension Water Or Oil Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Water, soapy water, or rubbing alcohol. Surface tension is the energy,. Which Has Higher Surface Tension Water Or Oil.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Which Has Higher Surface Tension Water Or Oil Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets Since these intermolecular forces vary depending on the nature of the. In this portion of the lab you will determine which liquid has the highest. Which Has Higher Surface Tension Water Or Oil.
From www.sciencephoto.com
Surface tension of water Stock Image C038/9387 Science Photo Library Which Has Higher Surface Tension Water Or Oil In this portion of the lab you will determine which liquid has the highest surface tension: Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. In order to do this, you will determine the number of droplets If a drop of water and oil is gently dropped on a flat surface, the. Which Has Higher Surface Tension Water Or Oil.
From ar.inspiredpencil.com
Surface Tension Water Which Has Higher Surface Tension Water Or Oil Water, soapy water, or rubbing alcohol. In this portion of the lab you will determine which liquid has the highest surface tension: Since these intermolecular forces vary depending on the nature of the. If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil. Which Has Higher Surface Tension Water Or Oil.
From news.mit.edu
MIT research on seawater surface tension international Which Has Higher Surface Tension Water Or Oil Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. In this portion of the lab you will. Which Has Higher Surface Tension Water Or Oil.
From ar.inspiredpencil.com
Surface Tension Water Which Has Higher Surface Tension Water Or Oil In order to do this, you will determine the number of droplets Since these intermolecular forces vary depending on the nature of the. Water has a high surface tension (72 dynes/cm). Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. If a drop of water and oil is gently dropped on a. Which Has Higher Surface Tension Water Or Oil.
From www.shutterstock.com
Insect Showing Water Surface Tension Stockvektor (royaltyfri Which Has Higher Surface Tension Water Or Oil Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In this portion of the lab you will determine which liquid has the highest surface tension: Since these intermolecular forces vary depending on the nature of the. If a drop of water and oil is gently dropped on a flat. Which Has Higher Surface Tension Water Or Oil.
From www.researchgate.net
(a) The interfacial tension between DI water and mineral oil (shown by Which Has Higher Surface Tension Water Or Oil If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water, soapy water, or rubbing alcohol. In this. Which Has Higher Surface Tension Water Or Oil.
From www.biolinscientific.com
Surface tension of water Why is it so high? Which Has Higher Surface Tension Water Or Oil If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. In this portion of the lab you will determine which liquid has the highest surface tension: Water, soapy water, or rubbing alcohol. Surface tension is the energy, or. Which Has Higher Surface Tension Water Or Oil.
From www.chegg.com
Solved Liquid X is known to have a higher surface tension Which Has Higher Surface Tension Water Or Oil Water, soapy water, or rubbing alcohol. Water has a high surface tension (72 dynes/cm). In order to do this, you will determine the number of droplets If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Since these. Which Has Higher Surface Tension Water Or Oil.
From www.chegg.com
Solved Which has a higher surface tension when liquid? i Which Has Higher Surface Tension Water Or Oil In order to do this, you will determine the number of droplets Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a high surface tension (72 dynes/cm). Since these intermolecular forces vary depending on the nature of the. If a drop of water and oil is gently. Which Has Higher Surface Tension Water Or Oil.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Which Has Higher Surface Tension Water Or Oil Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. In this portion of the lab you will determine which liquid has. Which Has Higher Surface Tension Water Or Oil.
From www.thoughtco.com
Liquid Definition in Chemistry Which Has Higher Surface Tension Water Or Oil In this portion of the lab you will determine which liquid has the highest surface tension: In order to do this, you will determine the number of droplets Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. If a drop of water and oil is gently dropped on a. Which Has Higher Surface Tension Water Or Oil.
From ar.inspiredpencil.com
Surface Tension Water Which Has Higher Surface Tension Water Or Oil Water, soapy water, or rubbing alcohol. If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. In order to do this, you will determine the number of droplets In this portion of the lab you will determine which. Which Has Higher Surface Tension Water Or Oil.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Which Has Higher Surface Tension Water Or Oil In order to do this, you will determine the number of droplets Water has a high surface tension (72 dynes/cm). Since these intermolecular forces vary depending on the nature of the. Water, soapy water, or rubbing alcohol. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. If a drop. Which Has Higher Surface Tension Water Or Oil.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse Which Has Higher Surface Tension Water Or Oil Water, soapy water, or rubbing alcohol. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Since these intermolecular forces vary depending on the nature of the. In order to do this, you will determine the number of droplets Water has a high surface tension (72 dynes/cm). In this portion of the lab. Which Has Higher Surface Tension Water Or Oil.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Which Has Higher Surface Tension Water Or Oil In this portion of the lab you will determine which liquid has the highest surface tension: Since these intermolecular forces vary depending on the nature of the. Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Water has a high surface tension (72 dynes/cm). In order to do this, you will determine. Which Has Higher Surface Tension Water Or Oil.
From www.biolinscientific.com
Why is surface tension important? Which Has Higher Surface Tension Water Or Oil Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. In this portion of the lab you will determine which liquid has the highest surface tension: Since these intermolecular forces vary depending on the nature of the. If a drop of water and oil is gently dropped on a flat surface, the droplet. Which Has Higher Surface Tension Water Or Oil.
From chem.libretexts.org
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts Which Has Higher Surface Tension Water Or Oil Water has a high surface tension (72 dynes/cm). Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the. In this portion of the lab you will determine which liquid has the highest surface tension: Water, soapy water, or rubbing. Which Has Higher Surface Tension Water Or Oil.
From megalitspb.ru
Подробно расскажем о Чем объясняется поверхностное натяжение воды Which Has Higher Surface Tension Water Or Oil Water has a high surface tension (72 dynes/cm). Water, soapy water, or rubbing alcohol. Since these intermolecular forces vary depending on the nature of the. If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. In this portion. Which Has Higher Surface Tension Water Or Oil.
From www.youtube.com
Surface Tension of Water Explained YouTube Which Has Higher Surface Tension Water Or Oil In this portion of the lab you will determine which liquid has the highest surface tension: Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a high surface tension (72. Which Has Higher Surface Tension Water Or Oil.
From inspiritvr.com
Surface Tension Study Guide Inspirit Learning Inc Which Has Higher Surface Tension Water Or Oil If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Water has a high surface tension (72 dynes/cm). In order to do this, you will determine the number of droplets In this portion of the lab you will. Which Has Higher Surface Tension Water Or Oil.
From www.scienceabc.com
Why Does Water Feel Like Concrete When You Belly Flop Into It? » ScienceABC Which Has Higher Surface Tension Water Or Oil Water has a high surface tension (72 dynes/cm). Water, soapy water, or rubbing alcohol. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In this portion of the lab you will determine which liquid has the highest surface. Which Has Higher Surface Tension Water Or Oil.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 Which Has Higher Surface Tension Water Or Oil Water, soapy water, or rubbing alcohol. In this portion of the lab you will determine which liquid has the highest surface tension: Since these intermolecular forces vary depending on the nature of the. Water has a high surface tension (72 dynes/cm). Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. Which Has Higher Surface Tension Water Or Oil.
From errantscience.com
ErrantScience Water surface tension Which Has Higher Surface Tension Water Or Oil If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Since these intermolecular forces vary depending on the nature of the. Water has a high surface tension (72 dynes/cm). Surface tension is the energy, or work, required to. Which Has Higher Surface Tension Water Or Oil.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID245553 Which Has Higher Surface Tension Water Or Oil If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. In this portion of the lab you will determine which liquid has the highest surface tension: Surface tension is the energy, or work, required to increase the surface. Which Has Higher Surface Tension Water Or Oil.
From bio1100.nicerweb.com
surface_tension/02_13 Which Has Higher Surface Tension Water Or Oil Liquids with high surface tension exhibit significant resistance to penetration compared to the resistance experienced in the. In order to do this, you will determine the number of droplets Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. Which Has Higher Surface Tension Water Or Oil.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Which Has Higher Surface Tension Water Or Oil If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. In this portion of the lab you will determine which liquid has the highest surface tension: In order to do this, you will determine the number of droplets. Which Has Higher Surface Tension Water Or Oil.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? Which Has Higher Surface Tension Water Or Oil If a drop of water and oil is gently dropped on a flat surface, the droplet of water will tend to be more spherical whereas the oil droplet will be a bit. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids with high surface tension exhibit significant resistance. Which Has Higher Surface Tension Water Or Oil.