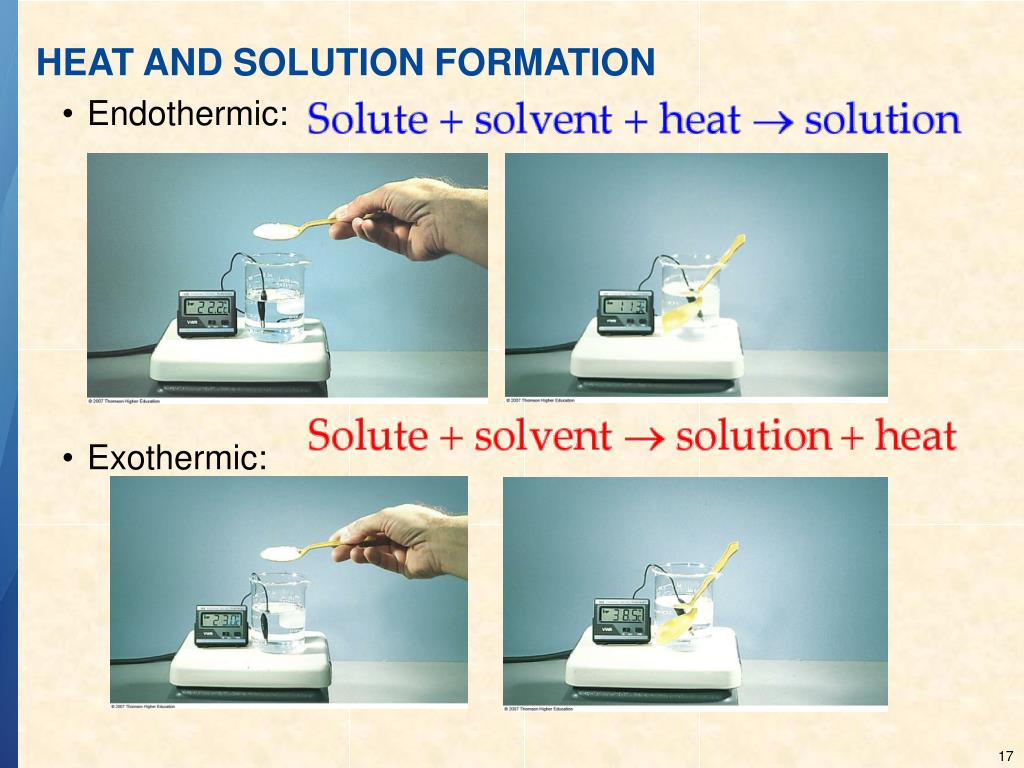
Endothermic Solution Formation Examples . Open systems are the most commonly encountered systems in. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. In both cases, step 1, separation of the solvent particles, is energetically uphill. Diagrammatic representations of the types of systems. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent.
from www.slideserve.com
Diagrammatic representations of the types of systems. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Open systems are the most commonly encountered systems in. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. An endothermic reaction is a chemical reaction that absorbs. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. In both cases, step 1, separation of the solvent particles, is energetically uphill. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs.
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free
Endothermic Solution Formation Examples Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Open systems are the most commonly encountered systems in. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. In both cases, step 1, separation of the solvent particles, is energetically uphill. Diagrammatic representations of the types of systems. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs.
From www.slideserve.com
PPT Endothermic and Exothermic PowerPoint Presentation, free download Endothermic Solution Formation Examples In both cases, step 1, separation of the solvent particles, is energetically uphill. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. Solvation can be an exothermic or endothermic process depending on the nature. Endothermic Solution Formation Examples.
From www.slideserve.com
PPT Heat from Reactions PowerPoint Presentation, free download ID Endothermic Solution Formation Examples Open systems are the most commonly encountered systems in. In both cases, step 1, separation of the solvent particles, is energetically uphill. Diagrammatic representations of the types of systems. An endothermic reaction is a chemical reaction that absorbs. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. For an endothermic chemical reaction to proceed,. Endothermic Solution Formation Examples.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Solution Formation Examples Open systems are the most commonly encountered systems in. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. When ammonium chloride (nh 4 cl) is dissolved in water, an. Endothermic Solution Formation Examples.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Solution Formation Examples When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a chemical reaction that absorbs. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous. Endothermic Solution Formation Examples.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID5403517 Endothermic Solution Formation Examples Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. An endothermic reaction is a chemical reaction that absorbs. Open systems are the most commonly encountered systems in. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Examples of endothermic reactions include photosynthesis, dissolving salt in water,. Endothermic Solution Formation Examples.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Solution Formation Examples An endothermic reaction is a chemical reaction that absorbs. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent.. Endothermic Solution Formation Examples.
From www.toppr.com
Differentiate between exothermic and endothermic reactions by giving Endothermic Solution Formation Examples Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Diagrammatic representations of the types of systems. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. An endothermic. Endothermic Solution Formation Examples.
From www.slideserve.com
PPT Solution Formation PowerPoint Presentation, free download ID Endothermic Solution Formation Examples When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a chemical reaction that absorbs. Diagrammatic representations of the types of systems. These examples illustrate how diffusion alone can provide the. Endothermic Solution Formation Examples.
From schempal.com
Understanding the Energy Level Diagram of an Endothermic Reaction Endothermic Solution Formation Examples Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. An endothermic reaction is a chemical reaction that absorbs. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs.. Endothermic Solution Formation Examples.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Solution Formation Examples In both cases, step 1, separation of the solvent particles, is energetically uphill. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Diagrammatic representations of the types of systems. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. These examples illustrate how diffusion alone. Endothermic Solution Formation Examples.
From education-portal.com
Endothermic Reaction Definition & Example Video & Lesson Transcript Endothermic Solution Formation Examples Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Diagrammatic representations of the types of systems. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. Open systems. Endothermic Solution Formation Examples.
From mavink.com
Draw An Energy Level Diagram For An Endothermic Reaction Endothermic Solution Formation Examples For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation. Endothermic Solution Formation Examples.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Solution Formation Examples Open systems are the most commonly encountered systems in. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation. Endothermic Solution Formation Examples.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID5403517 Endothermic Solution Formation Examples Diagrammatic representations of the types of systems. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Open systems are the most commonly encountered systems in. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. These examples illustrate how diffusion alone can provide the driving force required to cause the. Endothermic Solution Formation Examples.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Endothermic Solution Formation Examples For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Examples of endothermic reactions include. Endothermic Solution Formation Examples.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Solution Formation Examples For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. In both cases, step 1, separation of the solvent particles, is energetically uphill. An endothermic reaction is a chemical reaction that absorbs. Open systems are the most commonly encountered systems in. Solvation can be an exothermic or endothermic process depending. Endothermic Solution Formation Examples.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Endothermic Solution Formation Examples In both cases, step 1, separation of the solvent particles, is energetically uphill. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs. Diagrammatic representations of the types of systems. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation. Endothermic Solution Formation Examples.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Solution Formation Examples When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. In both cases, step 1, separation of the solvent particles, is energetically uphill. Diagrammatic representations of the types of systems. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. These examples illustrate how diffusion alone can provide the driving force. Endothermic Solution Formation Examples.
From adelynfvrush.blogspot.com
What is an Endothermic Reaction AdelynfvRush Endothermic Solution Formation Examples Diagrammatic representations of the types of systems. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. In both cases, step 1, separation of the solvent particles, is energetically uphill. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. An endothermic reaction is a. Endothermic Solution Formation Examples.
From www.slideserve.com
PPT Exo and Endothermic Reactions PowerPoint Presentation, free Endothermic Solution Formation Examples Open systems are the most commonly encountered systems in. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a chemical reaction that absorbs. Examples of endothermic reactions include photosynthesis, dissolving. Endothermic Solution Formation Examples.
From ar.inspiredpencil.com
Endothermic And Exothermic Reaction Examples Endothermic Solution Formation Examples For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Diagrammatic representations of the types of systems. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. An endothermic reaction is a chemical reaction that absorbs. Open systems are the most commonly encountered systems in.. Endothermic Solution Formation Examples.
From shaunmwilliams.com
Chapter 11 Presentation Endothermic Solution Formation Examples An endothermic reaction is a chemical reaction that absorbs. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. Diagrammatic representations of the types of systems. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Open systems are the most commonly encountered systems in.. Endothermic Solution Formation Examples.
From narodnatribuna.info
Endothermic Reactions Definition And Examples Endothermic Solution Formation Examples An endothermic reaction is a chemical reaction that absorbs. Diagrammatic representations of the types of systems. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Examples of endothermic reactions include photosynthesis, dissolving salt in. Endothermic Solution Formation Examples.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation ID Endothermic Solution Formation Examples Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. In both cases, step 1, separation of the solvent particles, is energetically uphill. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a chemical reaction that absorbs. Diagrammatic. Endothermic Solution Formation Examples.
From www.slideserve.com
PPT Exo and Endothermic Reactions PowerPoint Presentation, free Endothermic Solution Formation Examples Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. In both cases, step 1, separation of the solvent particles, is energetically uphill. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Diagrammatic. Endothermic Solution Formation Examples.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Solution Formation Examples For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. In both cases, step 1, separation of the solvent particles, is energetically uphill. An endothermic reaction is a chemical reaction that absorbs. Open systems are the most commonly encountered systems in. Solvation can be an exothermic or endothermic process depending. Endothermic Solution Formation Examples.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Endothermic Solution Formation Examples Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Open systems are the most commonly encountered systems in.. Endothermic Solution Formation Examples.
From guidewiringdietician.z5.web.core.windows.net
Enthalpy Diagram Endothermic Endothermic Solution Formation Examples When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. In both cases, step 1, separation of the solvent particles, is energetically uphill. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. An endothermic reaction is a chemical reaction that absorbs. Diagrammatic representations of. Endothermic Solution Formation Examples.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Endothermic Solution Formation Examples Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. In both cases, step 1, separation of the solvent particles, is energetically uphill. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction. Endothermic Solution Formation Examples.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Solution Formation Examples When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Open systems are the most commonly encountered systems in. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. In both cases, step 1, separation of the solvent particles, is energetically uphill. An endothermic reaction. Endothermic Solution Formation Examples.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Solution Formation Examples Diagrammatic representations of the types of systems. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. In both cases, step 1, separation of the solvent particles, is energetically uphill. Examples of endothermic reactions include. Endothermic Solution Formation Examples.
From www.slideserve.com
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free Endothermic Solution Formation Examples These examples illustrate how diffusion alone can provide the driving force required to cause the spontaneous formation of a solution. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Diagrammatic representations of. Endothermic Solution Formation Examples.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Solution Formation Examples Diagrammatic representations of the types of systems. Open systems are the most commonly encountered systems in. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. In both cases, step 1, separation of the solvent particles, is energetically uphill. An endothermic reaction is a chemical reaction that absorbs. Examples of endothermic reactions include. Endothermic Solution Formation Examples.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Solution Formation Examples Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. Open systems are the most commonly encountered systems in. In both cases, step 1, separation of the solvent particles, is energetically uphill. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Examples. Endothermic Solution Formation Examples.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Solution Formation Examples An endothermic reaction is a chemical reaction that absorbs. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. In both cases, step 1, separation of the solvent particles, is energetically uphill. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Open systems are the most commonly encountered systems in.. Endothermic Solution Formation Examples.