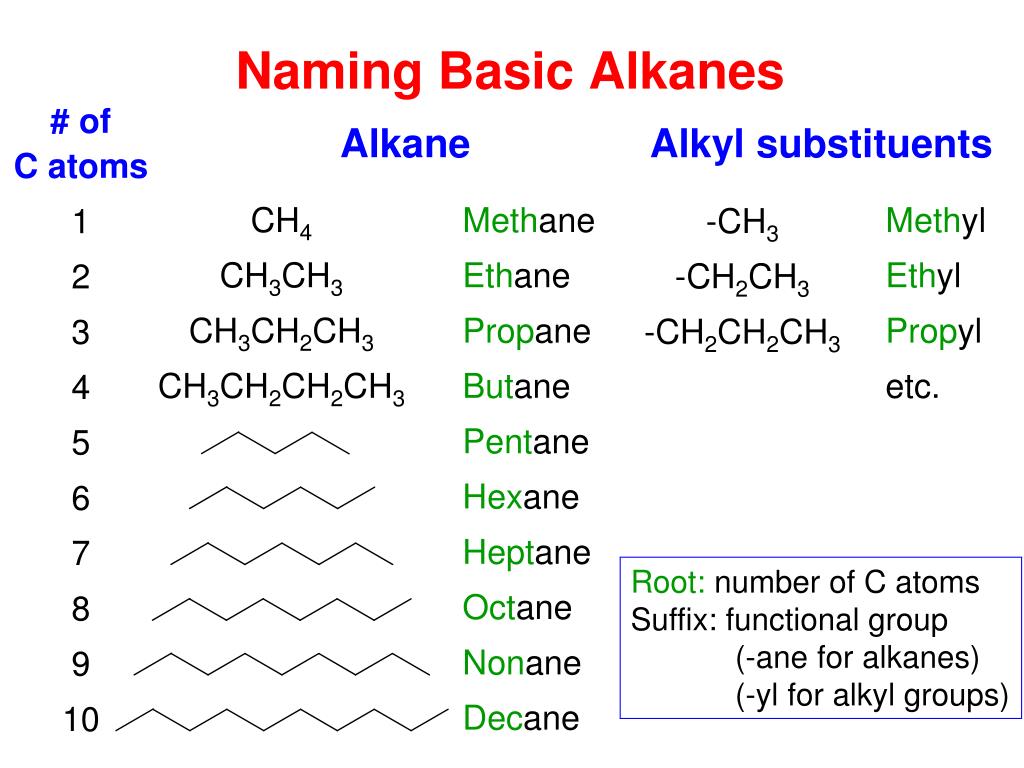
Is C4H10 Alkane Alkene Or Alkyne . Since c4h10 corresponds to the general. We have to classify each of the given molecular formula into alkane, alkene and alkyne. There are 4 steps to solve this one. Alkynes are also unsaturated hydrocarbons i.e. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. This leads to differences in geometries and in the hybridization of the carbon. Methane gas, whose molecular formula is ch4,.
from www.slideserve.com
We have to classify each of the given molecular formula into alkane, alkene and alkyne. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. There are 4 steps to solve this one. This leads to differences in geometries and in the hybridization of the carbon. Since c4h10 corresponds to the general. Methane gas, whose molecular formula is ch4,. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Alkenes and alkynes are two different classes of unsaturated hydrocarbons.
PPT Organic Chemistry PowerPoint Presentation, free download ID1945862
Is C4H10 Alkane Alkene Or Alkyne Alkenes and alkynes are two different classes of unsaturated hydrocarbons. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Methane gas, whose molecular formula is ch4,. There are 4 steps to solve this one. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. We have to classify each of the given molecular formula into alkane, alkene and alkyne. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. Since c4h10 corresponds to the general. Alkynes are also unsaturated hydrocarbons i.e. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. This leads to differences in geometries and in the hybridization of the carbon.
From www.shutterstock.com
Alkane Alkene Alkyne Functional Groups Organic Stock Illustration Is C4H10 Alkane Alkene Or Alkyne Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. This leads to differences in geometries and in the hybridization of the carbon. Alkynes are also unsaturated hydrocarbons i.e. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Since c4h10 corresponds to the general. There are 4 steps to solve. Is C4H10 Alkane Alkene Or Alkyne.
From ar.inspiredpencil.com
Alkane Alkene Alkyne Is C4H10 Alkane Alkene Or Alkyne Methane gas, whose molecular formula is ch4,. This leads to differences in geometries and in the hybridization of the carbon. Since c4h10 corresponds to the general. Alkynes are also unsaturated hydrocarbons i.e. There are 4 steps to solve this one. We have to classify each of the given molecular formula into alkane, alkene and alkyne. Methane (ch4), ethane (c2h6), propane. Is C4H10 Alkane Alkene Or Alkyne.
From gilbertorillooliver.blogspot.com
Alkane Alkene Alkyne GilbertorilloOliver Is C4H10 Alkane Alkene Or Alkyne Alkenes and alkynes are two different classes of unsaturated hydrocarbons. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. We have to classify each of the given molecular formula into alkane, alkene and alkyne. Since c4h10 corresponds to the general. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with.. Is C4H10 Alkane Alkene Or Alkyne.
From www.tes.com
Alkenes and Alkanes Edexcel IGCSE Chemistry Teaching Resources Is C4H10 Alkane Alkene Or Alkyne Since c4h10 corresponds to the general. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. We have to classify each of the given molecular formula into alkane, alkene and. Is C4H10 Alkane Alkene Or Alkyne.
From byjus.com
Alkanes Formula, Definition, Structure, Properties, Videos, List Is C4H10 Alkane Alkene Or Alkyne Since c4h10 corresponds to the general. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. This leads to differences in geometries and in the hybridization of the carbon. We have to classify each of the given molecular formula into alkane, alkene and alkyne. For. Is C4H10 Alkane Alkene Or Alkyne.
From www.studypool.com
SOLUTION Naming alkanes alkenes and alkynes Studypool Is C4H10 Alkane Alkene Or Alkyne Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. We have to classify each of the given molecular formula into alkane, alkene and alkyne. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. Since c4h10 corresponds to the general. This leads to differences in geometries and in. Is C4H10 Alkane Alkene Or Alkyne.
From www.chemistrysteps.com
Naming Alkynes by IUPAC Nomenclature Rules with Practice Problems Is C4H10 Alkane Alkene Or Alkyne Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. Methane gas, whose molecular formula is ch4,. Since c4h10 corresponds to the general. This leads to differences in geometries and in the hybridization of the carbon. Alkynes are also unsaturated hydrocarbons i.e. We have to classify each of the given molecular formula into alkane, alkene and. Is C4H10 Alkane Alkene Or Alkyne.
From slideplayer.com
Chapter 2 Alkanes. ppt download Is C4H10 Alkane Alkene Or Alkyne Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Alkynes are also unsaturated hydrocarbons i.e. Since c4h10 corresponds to the general. There are 4 steps to solve this one. This leads to differences in geometries and in the hybridization of. Is C4H10 Alkane Alkene Or Alkyne.
From www.slideserve.com
PPT Alkanes, Alkenes and Alkynes PowerPoint Presentation, free Is C4H10 Alkane Alkene Or Alkyne This leads to differences in geometries and in the hybridization of the carbon. Methane gas, whose molecular formula is ch4,. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. We have to classify each of the given molecular formula into alkane, alkene and alkyne. Alkenes and alkynes are two different classes of unsaturated hydrocarbons.. Is C4H10 Alkane Alkene Or Alkyne.
From www.toppr.com
Which compounds are called (i) alkanes, (ii) alkenes and (iii) alkynes Is C4H10 Alkane Alkene Or Alkyne For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Since c4h10 corresponds to the general. Alkynes are also unsaturated hydrocarbons i.e. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. We have to classify each of the given molecular formula into alkane, alkene and alkyne. Methane. Is C4H10 Alkane Alkene Or Alkyne.
From www.vedantu.com
Naming of Alkenes, Alkynes and Alkanes Important Concepts for JEE Is C4H10 Alkane Alkene Or Alkyne We have to classify each of the given molecular formula into alkane, alkene and alkyne. There are 4 steps to solve this one. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Methane (ch4), ethane (c2h6), propane (c3h8) and. Is C4H10 Alkane Alkene Or Alkyne.
From ar.inspiredpencil.com
C4h10 Isomers How Many Is C4H10 Alkane Alkene Or Alkyne Since c4h10 corresponds to the general. This leads to differences in geometries and in the hybridization of the carbon. Methane gas, whose molecular formula is ch4,. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Alkynes are also unsaturated hydrocarbons i.e. We have to classify each of the given molecular formula into alkane, alkene. Is C4H10 Alkane Alkene Or Alkyne.
From www.youtube.com
Differences Between Alkane, Alkene, and Alkyne. YouTube Is C4H10 Alkane Alkene Or Alkyne For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. Alkynes are also unsaturated hydrocarbons i.e. This leads to differences in geometries and. Is C4H10 Alkane Alkene Or Alkyne.
From slideplayer.com
Chapter 2 Alkanes. ppt download Is C4H10 Alkane Alkene Or Alkyne For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Since c4h10 corresponds to the general. This leads to differences in geometries and in the hybridization of the carbon. We have to classify each of the given molecular formula into alkane, alkene and alkyne. Alkynes are also unsaturated hydrocarbons i.e. Methane gas, whose molecular formula. Is C4H10 Alkane Alkene Or Alkyne.
From mavink.com
Naming Alkanes Chart Is C4H10 Alkane Alkene Or Alkyne We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. There are 4 steps to solve this one. We have to classify each of the given molecular formula into alkane,. Is C4H10 Alkane Alkene Or Alkyne.
From brainly.com
Question 1 Alkanes, Alkenes, and Alkynes Complete the table by Is C4H10 Alkane Alkene Or Alkyne There are 4 steps to solve this one. Since c4h10 corresponds to the general. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Alkynes are also unsaturated. Is C4H10 Alkane Alkene Or Alkyne.
From www.studocu.com
Alkane, alkene, alkyne LECTURE 1. ALKANES (PARAFFINS), ALKENES Is C4H10 Alkane Alkene Or Alkyne Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. Since c4h10 corresponds to the general. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Alkenes and alkynes are two different classes of. Is C4H10 Alkane Alkene Or Alkyne.
From byjus.com
How is structure of Alkynes are different from that of Alkenes? Is C4H10 Alkane Alkene Or Alkyne Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. This leads to differences in geometries and in the hybridization of the carbon. Methane gas, whose molecular formula is ch4,. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. Alkynes are also unsaturated hydrocarbons i.e. We can distinguish. Is C4H10 Alkane Alkene Or Alkyne.
From www.chemistrysteps.com
Naming Alkynes by IUPAC Nomenclature Rules with Practice Problems Is C4H10 Alkane Alkene Or Alkyne For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. This leads to differences in geometries and in the hybridization of the carbon. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule.. Is C4H10 Alkane Alkene Or Alkyne.
From www.aceorganicchem.com
Alkane Formula [with free study guide] Is C4H10 Alkane Alkene Or Alkyne We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. We have to classify each of the given molecular formula into alkane, alkene and alkyne. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. Since c4h10 corresponds to the general.. Is C4H10 Alkane Alkene Or Alkyne.
From slideplayer.com
Organic Chemistry Structural isomers Naming Branched Alkanes. ppt Is C4H10 Alkane Alkene Or Alkyne Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. Methane gas, whose molecular formula is ch4,. Since c4h10 corresponds to the general. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. We have to classify each of the given molecular formula into alkane, alkene and alkyne. For. Is C4H10 Alkane Alkene Or Alkyne.
From www.studypool.com
SOLUTION Alkane alkene and alkynes chemistry Studypool Is C4H10 Alkane Alkene Or Alkyne This leads to differences in geometries and in the hybridization of the carbon. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. Alkynes are also unsaturated hydrocarbons i.e. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. We have to classify each of the given molecular formula into alkane, alkene and alkyne. There are. Is C4H10 Alkane Alkene Or Alkyne.
From wou.edu
CH105 Chapter 7 Alkanes and Halogenated Hydrocarbons Chemistry Is C4H10 Alkane Alkene Or Alkyne Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. Since c4h10 corresponds to the general. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. This leads to differences in geometries and in the hybridization of the carbon.. Is C4H10 Alkane Alkene Or Alkyne.
From www.vedantu.com
Naming of Alkenes, Alkynes and Alkanes Important Concepts for JEE Is C4H10 Alkane Alkene Or Alkyne Methane gas, whose molecular formula is ch4,. This leads to differences in geometries and in the hybridization of the carbon. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. Since c4h10 corresponds to the general. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. For example, if \(n=2,\) the. Is C4H10 Alkane Alkene Or Alkyne.
From www.youtube.com
C4H10 Lewis Structure How to Draw the Lewis Structure for C4H10 YouTube Is C4H10 Alkane Alkene Or Alkyne Alkynes are also unsaturated hydrocarbons i.e. There are 4 steps to solve this one. Methane gas, whose molecular formula is ch4,. We have to classify each of the given molecular formula into alkane, alkene and alkyne. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Alkenes and alkynes are two different classes of unsaturated. Is C4H10 Alkane Alkene Or Alkyne.
From saylordotorg.github.io
Structures and Names of Alkanes Is C4H10 Alkane Alkene Or Alkyne Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. Alkynes are also unsaturated hydrocarbons i.e. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. This leads to differences in geometries and in the hybridization of the carbon. Methane gas, whose molecular formula is ch4,. Methane (ch4),. Is C4H10 Alkane Alkene Or Alkyne.
From www.chegg.com
Solved 2 Isomers of Alkanes Isomers of C4H10 2Methylpropane Is C4H10 Alkane Alkene Or Alkyne Since c4h10 corresponds to the general. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. There are 4 steps to solve this one. This leads to differences in geometries and in the hybridization of the carbon. Methane gas, whose molecular formula is ch4,. We can distinguish several types of hydrocarbons by differences in the. Is C4H10 Alkane Alkene Or Alkyne.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID1945862 Is C4H10 Alkane Alkene Or Alkyne Since c4h10 corresponds to the general. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. Methane gas, whose molecular formula is ch4,. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms.. Is C4H10 Alkane Alkene Or Alkyne.
From owlcation.com
The Chemistry of Alkenes Structure, Naming, Uses & Reactions Owlcation Is C4H10 Alkane Alkene Or Alkyne There are 4 steps to solve this one. We have to classify each of the given molecular formula into alkane, alkene and alkyne. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Since c4h10 corresponds to the general. This. Is C4H10 Alkane Alkene Or Alkyne.
From barrygraygillingham.com
A simple introduction to organic chemistry Is C4H10 Alkane Alkene Or Alkyne Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Methane gas, whose molecular formula is ch4,. Alkenes and alkynes are two different classes of unsaturated hydrocarbons. For example, if \(n=2,\) the alkene is called as ethene or. Is C4H10 Alkane Alkene Or Alkyne.
From www.slideshare.net
Alkane,alkene,alkyne Is C4H10 Alkane Alkene Or Alkyne Methane gas, whose molecular formula is ch4,. Alkynes are also unsaturated hydrocarbons i.e. For example, if \(n=2,\) the alkene is called as ethene or also as ethylene with. This leads to differences in geometries and in the hybridization of the carbon. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. We have to classify each. Is C4H10 Alkane Alkene Or Alkyne.
From chemistnotes.com
Alkenes formula, structure, nomenclature, properties, and uses Is C4H10 Alkane Alkene Or Alkyne We have to classify each of the given molecular formula into alkane, alkene and alkyne. This leads to differences in geometries and in the hybridization of the carbon. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. There are 4 steps to solve this one. Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10). Is C4H10 Alkane Alkene Or Alkyne.
From www.youtube.com
Alkanes, Alkenes, and Alkynes General molecular formula Chemistry Is C4H10 Alkane Alkene Or Alkyne Alkynes are also unsaturated hydrocarbons i.e. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. We have to classify each of the given molecular formula into alkane, alkene and alkyne. Methane gas, whose molecular formula is ch4,. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms.. Is C4H10 Alkane Alkene Or Alkyne.
From brainly.in
Which compounds are called (i) alkanes, (ii) alkenes and (iii) alkynes Is C4H10 Alkane Alkene Or Alkyne Methane (ch4), ethane (c2h6), propane (c3h8) and butane (c4h10) are the first four alkanes. There are 4 steps to solve this one. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. Since c4h10 corresponds to the general. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms.. Is C4H10 Alkane Alkene Or Alkyne.
From quizlet.com
Naming Alkanes, Alkenes, and Alkynes Diagram Quizlet Is C4H10 Alkane Alkene Or Alkyne We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. There are 4 steps to solve this one. This leads to differences in geometries and in the hybridization of the carbon. We have to classify each of the given molecular formula into alkane, alkene and alkyne. Alkenes have the general formula \(\ce{c_nh_{2n}},\) where \(n\) is. Is C4H10 Alkane Alkene Or Alkyne.