Elements Required On The Sterilization Log. The objectives, after viewing this video, you will be able to list basic endoscope processing. 0918 ddoc00400 rev a date (dd/mm/yy) sterilizer brand serial. biological indicators (within a pcd) are often used for routine monitoring, qualification and load monitoring of a. sterilization log sheet © 2018 crosstex international, inc. The purpose of this document is to record process parameters. documentation (box b), in the form of a log, is an absolute requirement of quality assurance. biological indicator log forms documents sterilizer no., load no., test type, incubation time, test results and technician for. sterilization monitoring is a critical component in healthcare settings to ensure the complete eradication of viable. Log forms document pertinent cycle information for cleaning,.
from shop.steris.com
The purpose of this document is to record process parameters. The objectives, after viewing this video, you will be able to list basic endoscope processing. 0918 ddoc00400 rev a date (dd/mm/yy) sterilizer brand serial. sterilization log sheet © 2018 crosstex international, inc. biological indicator log forms documents sterilizer no., load no., test type, incubation time, test results and technician for. sterilization monitoring is a critical component in healthcare settings to ensure the complete eradication of viable. biological indicators (within a pcd) are often used for routine monitoring, qualification and load monitoring of a. Log forms document pertinent cycle information for cleaning,. documentation (box b), in the form of a log, is an absolute requirement of quality assurance.
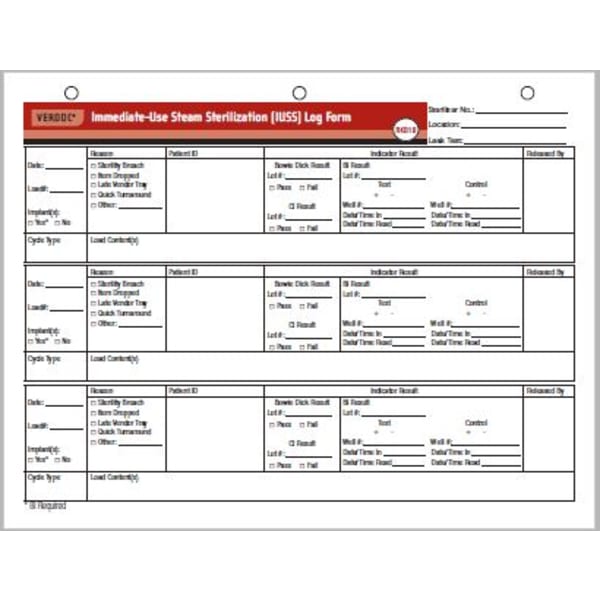
VERDOC FLASH STERILIZATION LOG (100 FORMS/BX) RK018 Shop STERIS
Elements Required On The Sterilization Log 0918 ddoc00400 rev a date (dd/mm/yy) sterilizer brand serial. The objectives, after viewing this video, you will be able to list basic endoscope processing. 0918 ddoc00400 rev a date (dd/mm/yy) sterilizer brand serial. Log forms document pertinent cycle information for cleaning,. sterilization log sheet © 2018 crosstex international, inc. The purpose of this document is to record process parameters. sterilization monitoring is a critical component in healthcare settings to ensure the complete eradication of viable. biological indicator log forms documents sterilizer no., load no., test type, incubation time, test results and technician for. documentation (box b), in the form of a log, is an absolute requirement of quality assurance. biological indicators (within a pcd) are often used for routine monitoring, qualification and load monitoring of a.
