Boron Fluoride Cation And Anion . An ionic compound is never formed between two cations only or two. a proper ionic formula has a cation and an anion in it; many ionic compounds contain polyatomic ions as the cation, the anion, or both. the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. All the anions are of this type, gaining the number. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. As with simple ionic compounds, these. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative.
from www.youtube.com
— with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. a proper ionic formula has a cation and an anion in it; — we have also considered cationic boranes, which form chelate complexes with fluoride anions. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. As with simple ionic compounds, these. the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. All the anions are of this type, gaining the number. many ionic compounds contain polyatomic ions as the cation, the anion, or both. An ionic compound is never formed between two cations only or two.
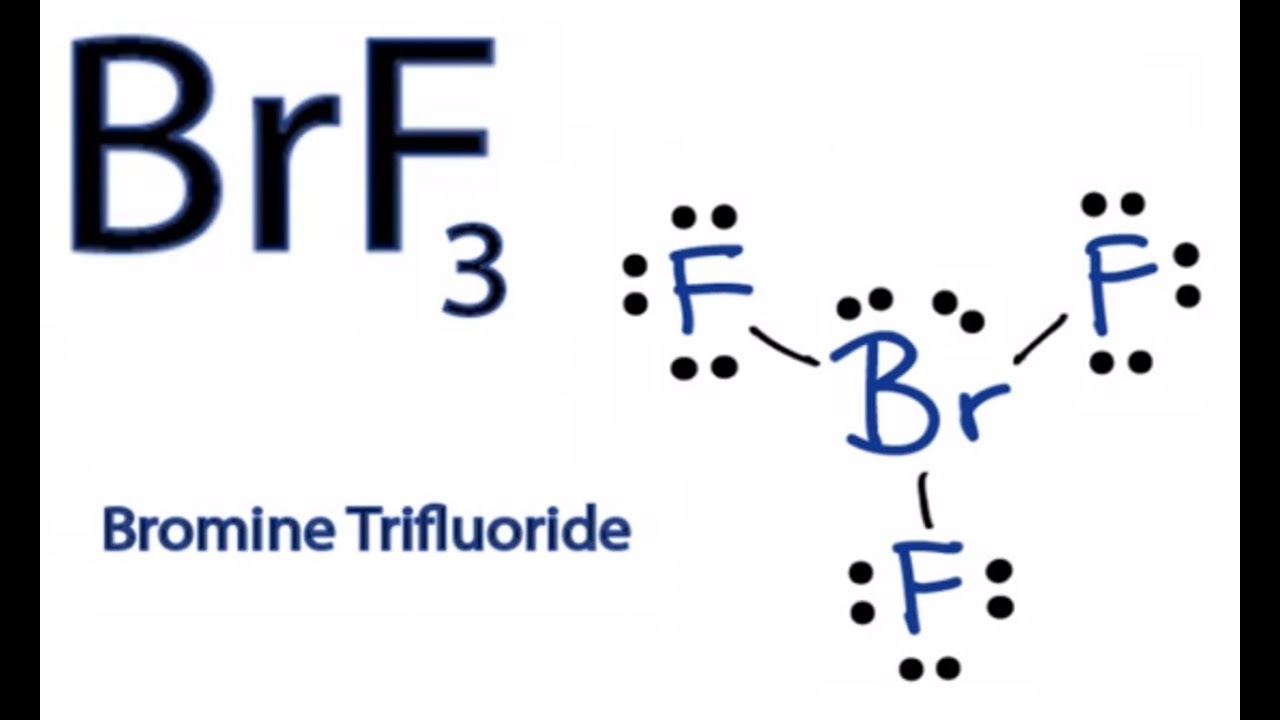
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube
Boron Fluoride Cation And Anion figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. An ionic compound is never formed between two cations only or two. many ionic compounds contain polyatomic ions as the cation, the anion, or both. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. All the anions are of this type, gaining the number. As with simple ionic compounds, these. a proper ionic formula has a cation and an anion in it;
From www.aquaportail.com
Anion définition et explications Boron Fluoride Cation And Anion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. An ionic compound is never formed between two cations only or two. All the anions are of this type, gaining the number. a proper ionic formula has a cation and an anion in it;. Boron Fluoride Cation And Anion.
From www.researchgate.net
Anionic species with boroncentred nucleophilicity. Cations omitted Boron Fluoride Cation And Anion An ionic compound is never formed between two cations only or two. All the anions are of this type, gaining the number. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. many ionic compounds contain polyatomic ions as the cation, the anion, or both. the formula unit or empirical formula represents the. Boron Fluoride Cation And Anion.
From byjus.com
Explain why the molecular shape of boron trifluoride is trigonal planar. Boron Fluoride Cation And Anion figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. a proper ionic formula has a cation and an anion in it; All the anions are of this type, gaining the number. An ionic compound is never formed between two cations only or two. — we have also considered cationic boranes, which form chelate. Boron Fluoride Cation And Anion.
From www.bigstockphoto.com
BF3 Boron Fluoride Vector & Photo (Free Trial) Bigstock Boron Fluoride Cation And Anion a proper ionic formula has a cation and an anion in it; All the anions are of this type, gaining the number. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. As with simple ionic compounds, these. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. An ionic. Boron Fluoride Cation And Anion.
From slideplayer.com
Unit 6 Covalent Bonding ppt download Boron Fluoride Cation And Anion — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. All the anions are of this type, gaining the number. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. An ionic compound is. Boron Fluoride Cation And Anion.
From stock.adobe.com
Stylized molecule model/structural formula of boron trifluoride. Stock Boron Fluoride Cation And Anion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. As with simple ionic compounds, these. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states.. Boron Fluoride Cation And Anion.
From www.youtube.com
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube Boron Fluoride Cation And Anion the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. a proper ionic formula has a cation and an anion in it; figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. many ionic compounds contain polyatomic ions as the cation, the anion, or both. All. Boron Fluoride Cation And Anion.
From chem.libretexts.org
11.2 Lewis Symbols and Structures Chemistry LibreTexts Boron Fluoride Cation And Anion figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. All the anions are of this type, gaining the number. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its. Boron Fluoride Cation And Anion.
From dauglas.afphila.com
Cations and Anions Difference between Cations and Anions Boron Fluoride Cation And Anion — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. many ionic compounds contain polyatomic ions as the cation,. Boron Fluoride Cation And Anion.
From mavink.com
Common Anions And Cations Table Boron Fluoride Cation And Anion the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. All the anions are of this type, gaining the number. many ionic compounds contain polyatomic ions as the cation, the anion, or both. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. a cation. Boron Fluoride Cation And Anion.
From www.researchgate.net
RDFs of cationanion and anionanion pairs for the fluoride ion Boron Fluoride Cation And Anion many ionic compounds contain polyatomic ions as the cation, the anion, or both. An ionic compound is never formed between two cations only or two. As with simple ionic compounds, these. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. a proper ionic formula has a cation and an anion in it; . Boron Fluoride Cation And Anion.
From adrienj.tinosmarble.com
Cations vs Anions Difference Between Cations and Anions with Examples Boron Fluoride Cation And Anion All the anions are of this type, gaining the number. An ionic compound is never formed between two cations only or two. the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. figure 2.7.2 lists the. Boron Fluoride Cation And Anion.
From www.researchgate.net
Boroncentered stable radical anion/radical cation pair. Reproduced Boron Fluoride Cation And Anion figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative.. Boron Fluoride Cation And Anion.
From trailerspotting.blogspot.com
40 lewis electron dot diagram for fluoride ion Boron Fluoride Cation And Anion a proper ionic formula has a cation and an anion in it; figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type, gaining the number. As with simple ionic compounds, these. the formula unit or empirical formula represents the minimum proportion between cations and anions in the. Boron Fluoride Cation And Anion.
From www.difference101.com
Cation vs. Anion 7 Key Differences, Pros & Cons, Examples Difference 101 Boron Fluoride Cation And Anion — we have also considered cationic boranes, which form chelate complexes with fluoride anions. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. a proper ionic formula has. Boron Fluoride Cation And Anion.
From www.vectorstock.com
Bf3 boron fluoride molecule Royalty Free Vector Image Boron Fluoride Cation And Anion As with simple ionic compounds, these. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. An ionic compound is never formed between two cations only or two. All the anions. Boron Fluoride Cation And Anion.
From chemistry-europe.onlinelibrary.wiley.com
Anion‐Dependent Reactivity of Mono‐ and Dinuclear Boron Cations Boron Fluoride Cation And Anion — we have also considered cationic boranes, which form chelate complexes with fluoride anions. the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. a proper ionic formula has a cation and an anion in it; figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states.. Boron Fluoride Cation And Anion.
From www.researchgate.net
Multistep hydrolysis of boron trifluoride leading to boric acid and Boron Fluoride Cation And Anion — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. All the anions are of this type, gaining the number. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. As with simple ionic. Boron Fluoride Cation And Anion.
From www.scribd.com
Common Ions Anions and Cations PDF Boron Fluoride Cation And Anion the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. As with simple ionic compounds, these. figure 2.7.2 lists the ions (cation and anion) that have. Boron Fluoride Cation And Anion.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Boron Fluoride Cation And Anion figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. many ionic compounds contain polyatomic ions as the cation, the anion, or both. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. As with simple ionic compounds, these. An. Boron Fluoride Cation And Anion.
From slideplayer.com
Chapter 4 Skills Balance Equations for simple chemical reactions ppt Boron Fluoride Cation And Anion — we have also considered cationic boranes, which form chelate complexes with fluoride anions. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. many. Boron Fluoride Cation And Anion.
From slideplayer.com
Chapter 5 Online Resource ppt download Boron Fluoride Cation And Anion All the anions are of this type, gaining the number. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. An ionic compound is never formed between two cations only or two. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. many ionic. Boron Fluoride Cation And Anion.
From pdfslide.net
(Download PPTX Powerpoint) Naming IONS & formulas for Ionic Compounds Boron Fluoride Cation And Anion All the anions are of this type, gaining the number. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. the formula unit or empirical formula represents the minimum proportion between cations and. Boron Fluoride Cation And Anion.
From www.semanticscholar.org
Figure 1 from Boron as a powerful reductant synthesis of a stable Boron Fluoride Cation And Anion figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type, gaining the number. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. An ionic compound is never formed between two cations only or two. a proper. Boron Fluoride Cation And Anion.
From brainly.com
Complete the equation for the Lewis acid/Lewis base reaction between Boron Fluoride Cation And Anion An ionic compound is never formed between two cations only or two. All the anions are of this type, gaining the number. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. As with. Boron Fluoride Cation And Anion.
From www.researchgate.net
Structural illustration of boron based anions (a) BMdB, (b) BScB, (c Boron Fluoride Cation And Anion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. — we have also considered cationic boranes, which form chelate complexes with fluoride anions. An ionic compound is never formed between two cations only or two. As with simple ionic compounds, these. figure. Boron Fluoride Cation And Anion.
From slideplayer.com
Groundwater Quality UNIT ppt download Boron Fluoride Cation And Anion — we have also considered cationic boranes, which form chelate complexes with fluoride anions. All the anions are of this type, gaining the number. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. many ionic compounds contain polyatomic ions as the cation,. Boron Fluoride Cation And Anion.
From www.researchgate.net
Optimized anion geometries of normal heterocyclic systems with fluoride Boron Fluoride Cation And Anion As with simple ionic compounds, these. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. An ionic compound is never formed between two cations only or two. the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. — we have also considered cationic boranes, which form. Boron Fluoride Cation And Anion.
From www.researchgate.net
Coordination sphere around one [Me 3 NB 12 Cl 11 ] À anion. The boron Boron Fluoride Cation And Anion An ionic compound is never formed between two cations only or two. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. many ionic compounds contain polyatomic ions as the cation, the anion, or both. figure 2.7.2 lists the ions (cation and anion). Boron Fluoride Cation And Anion.
From www.difference101.com
Cation vs. Anion 7 Key Differences, Pros & Cons, Examples Difference 101 Boron Fluoride Cation And Anion figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. An ionic compound is never formed between two cations only or two. many ionic compounds contain polyatomic ions as the cation, the anion, or both. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the. Boron Fluoride Cation And Anion.
From www.dreamstime.com
Cations and Anions. Structure of Ions Stock Vector Illustration of Boron Fluoride Cation And Anion — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. As with simple ionic compounds, these. many ionic compounds contain polyatomic ions as the cation, the anion, or both. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence. Boron Fluoride Cation And Anion.
From robot.ekstrabladet.dk
Cátions E ânions Tabela Boron Fluoride Cation And Anion many ionic compounds contain polyatomic ions as the cation, the anion, or both. figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. a proper ionic formula has a cation and an anion in it; — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in. Boron Fluoride Cation And Anion.
From www.solvedlib.com
Draw the detailed mechanism for the reaction between … SolvedLib Boron Fluoride Cation And Anion — we have also considered cationic boranes, which form chelate complexes with fluoride anions. a proper ionic formula has a cation and an anion in it; An ionic compound is never formed between two cations only or two. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the. Boron Fluoride Cation And Anion.
From slideplayer.com
Binary Compounds NaCl sodium chlor ine ide (Na1+ Cl1) CaS ppt download Boron Fluoride Cation And Anion the formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. a proper ionic formula has a cation and an anion in it; An ionic compound is. Boron Fluoride Cation And Anion.
From slideplayer.com
Chapter 5 Nomenclature. ppt download Boron Fluoride Cation And Anion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. — with the exception of systems bearing bodipy dyes, where the boron centre is not directly involved in the interaction. As with simple ionic compounds, these. An ionic compound is never formed between two. Boron Fluoride Cation And Anion.