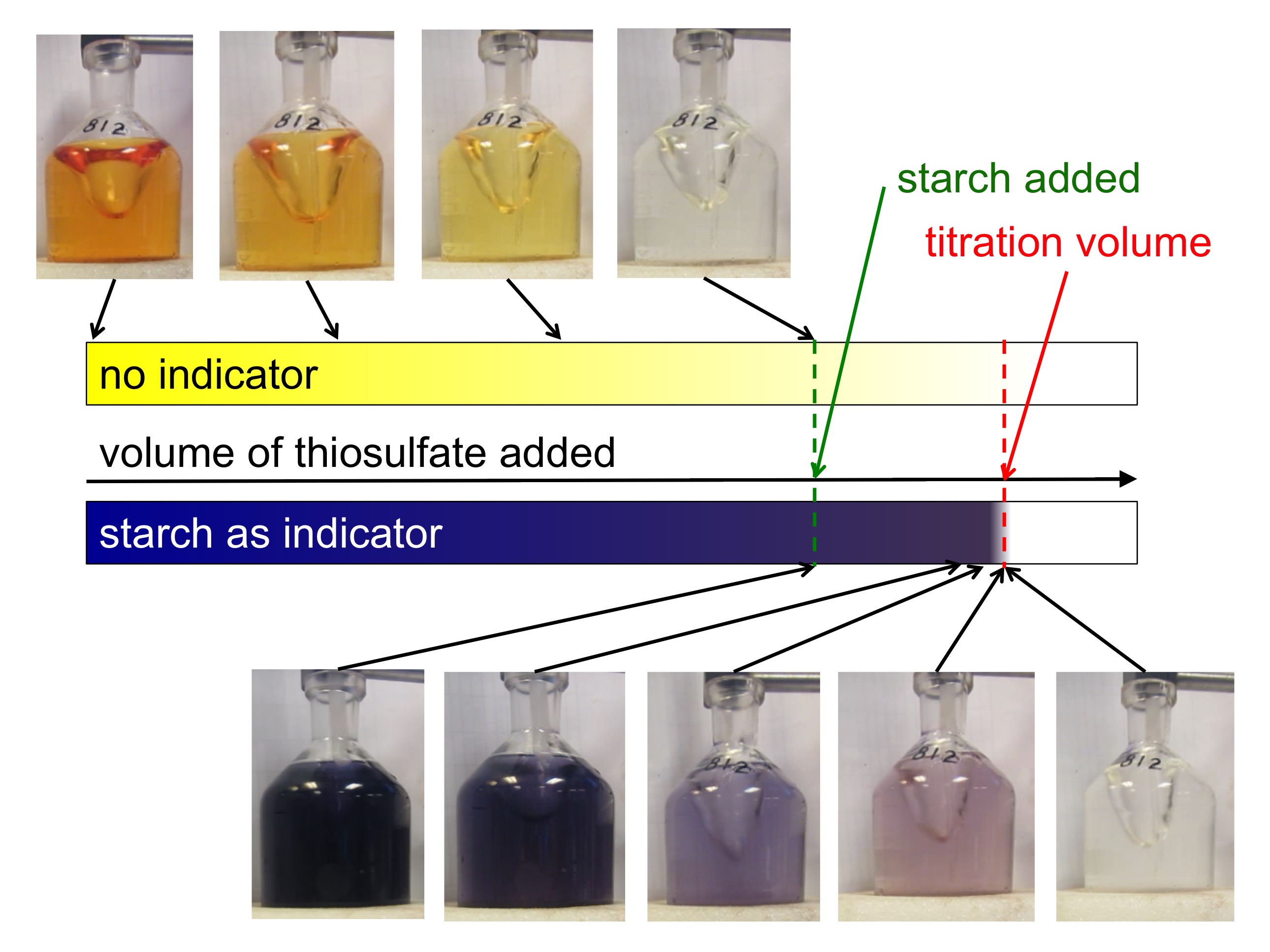
End Point For A Titration . a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. Figure caption, phenolphthalein is pink in. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: finding the endpoint of a titration.
from exoofexjf.blob.core.windows.net
— a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. \ce{naoh}\) is required to reach the end. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. Figure caption, phenolphthalein is pink in. finding the endpoint of a titration. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. suppose that a titration is performed and \(20.70 \:
End Point Color Titration at Charles Vesey blog
End Point For A Titration For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Figure caption, phenolphthalein is pink in. — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: suppose that a titration is performed and \(20.70 \: Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. finding the endpoint of a titration. \ce{naoh}\) is required to reach the end. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample.
From mungfali.com
Endpoint Titration Curve End Point For A Titration finding the endpoint of a titration. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. For a ph titration (between an acid and a base), there are two common ways to. End Point For A Titration.
From www.animalia-life.club
Endpoint Titration End Point For A Titration Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. finding the endpoint of a titration. \ce{naoh}\) is required to reach the end. . End Point For A Titration.
From www.animalia-life.club
Endpoint Titration End Point For A Titration — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: \ce{naoh}\) is required to reach the end. finding the endpoint of a titration. Involves the neutralization of an. End Point For A Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts End Point For A Titration — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: — a titration’s end point was determined using litmus as an indicator, which is red. End Point For A Titration.
From ar.inspiredpencil.com
Endpoint Titration End Point For A Titration a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. — for your first titration, add titrant about one ml at a time to get a. End Point For A Titration.
From www.animalia-life.club
Endpoint Titration End Point For A Titration For a ph titration (between an acid and a base), there are two common ways to find the endpoint: — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a point of equivalence in a titration refers to a point at which. End Point For A Titration.
From slideplayer.com
Titrations!. ppt download End Point For A Titration a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. — a titration’s end point was determined using litmus as an. End Point For A Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point For A Titration \ce{naoh}\) is required to reach the end. Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. For a ph titration (between an acid and. End Point For A Titration.
From www.animalia-life.club
Endpoint Titration End Point For A Titration suppose that a titration is performed and \(20.70 \: finding the endpoint of a titration. Figure caption, phenolphthalein is pink in. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. — a titration is a volumetric technique in which a solution of one. End Point For A Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent End Point For A Titration suppose that a titration is performed and \(20.70 \: — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end. End Point For A Titration.
From ar.inspiredpencil.com
Endpoint Titration End Point For A Titration a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. \ce{naoh}\) is required to reach the end. finding the endpoint of a titration. Involves. End Point For A Titration.
From www.animalia-life.club
Endpoint Titration End Point For A Titration — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. suppose that a titration is performed and \(20.70 \: — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. For. End Point For A Titration.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog End Point For A Titration \ce{naoh}\) is required to reach the end. Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. suppose that a titration is performed and \(20.70 \: — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. . End Point For A Titration.
From ar.inspiredpencil.com
Endpoint Titration End Point For A Titration suppose that a titration is performed and \(20.70 \: Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: — a titration is a volumetric technique in which a. End Point For A Titration.
From www.animalia-life.club
Endpoint Titration End Point For A Titration — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. suppose that a titration is performed and \(20.70 \:. End Point For A Titration.
From www.animalia-life.club
Endpoint Titration End Point For A Titration \ce{naoh}\) is required to reach the end. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. — a titration’s end point was determined using litmus as. End Point For A Titration.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog End Point For A Titration — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. finding the endpoint of a titration. Figure caption, phenolphthalein is. End Point For A Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog End Point For A Titration \ce{naoh}\) is required to reach the end. Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. For a ph titration (between an acid and a base),. End Point For A Titration.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder End Point For A Titration Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: suppose that a titration is performed and \(20.70 \: Figure caption, phenolphthalein is pink in. — a titration is. End Point For A Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent End Point For A Titration a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. \ce{naoh}\) is required to reach the end. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. suppose that. End Point For A Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts End Point For A Titration — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. \ce{naoh}\) is required to reach the end. Figure caption, phenolphthalein is pink in.. End Point For A Titration.
From www.numerade.com
SOLVED 3. Use a chemical dictionary or encyclopedia to explain the End Point For A Titration Figure caption, phenolphthalein is pink in. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. — for your. End Point For A Titration.
From exovodadv.blob.core.windows.net
Titration End Point Meaning at Jenny Ingram blog End Point For A Titration \ce{naoh}\) is required to reach the end. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Figure caption, phenolphthalein is pink in. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent. End Point For A Titration.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts End Point For A Titration — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. . End Point For A Titration.
From exoofexjf.blob.core.windows.net
End Point Color Titration at Charles Vesey blog End Point For A Titration For a ph titration (between an acid and a base), there are two common ways to find the endpoint: a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. \ce{naoh}\) is required to reach the end. Figure caption, phenolphthalein is pink in. finding the endpoint of. End Point For A Titration.
From www.animalia-life.club
Endpoint Titration End Point For A Titration finding the endpoint of a titration. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. — a titration is a volumetric technique. End Point For A Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators End Point For A Titration — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Involves the neutralization of an acid with a base (or vice versa) using a ph indicator. End Point For A Titration.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog End Point For A Titration suppose that a titration is performed and \(20.70 \: — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. a point of equivalence in a titration. End Point For A Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts End Point For A Titration — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: finding the endpoint of a titration. suppose that a titration is performed and \(20.70. End Point For A Titration.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center End Point For A Titration Figure caption, phenolphthalein is pink in. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. suppose that a titration is performed and \(20.70 \:. End Point For A Titration.
From animalia-life.club
Endpoint Titration End Point For A Titration Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. \ce{naoh}\) is required to reach the end. — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. suppose that a titration is performed and \(20.70 \: For. End Point For A Titration.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog End Point For A Titration — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. Figure caption, phenolphthalein is pink in. finding the endpoint of a titration. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: — a. End Point For A Titration.
From theedge.com.hk
Chemistry How To Titration The Edge End Point For A Titration suppose that a titration is performed and \(20.70 \: — a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. — for your first titration, add titrant about one ml at a time to get a rough idea of where the end point is. — a. End Point For A Titration.
From www.animalia-life.club
Endpoint Titration End Point For A Titration Figure caption, phenolphthalein is pink in. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Involves the neutralization of. End Point For A Titration.
From www.chemiepedia.nl
Wat is het eindpunt van een titratie? Chemiepedia.nl End Point For A Titration Involves the neutralization of an acid with a base (or vice versa) using a ph indicator to determine the endpoint. \ce{naoh}\) is required to reach the end. Figure caption, phenolphthalein is pink in. suppose that a titration is performed and \(20.70 \: For a ph titration (between an acid and a base), there are two common ways to find. End Point For A Titration.