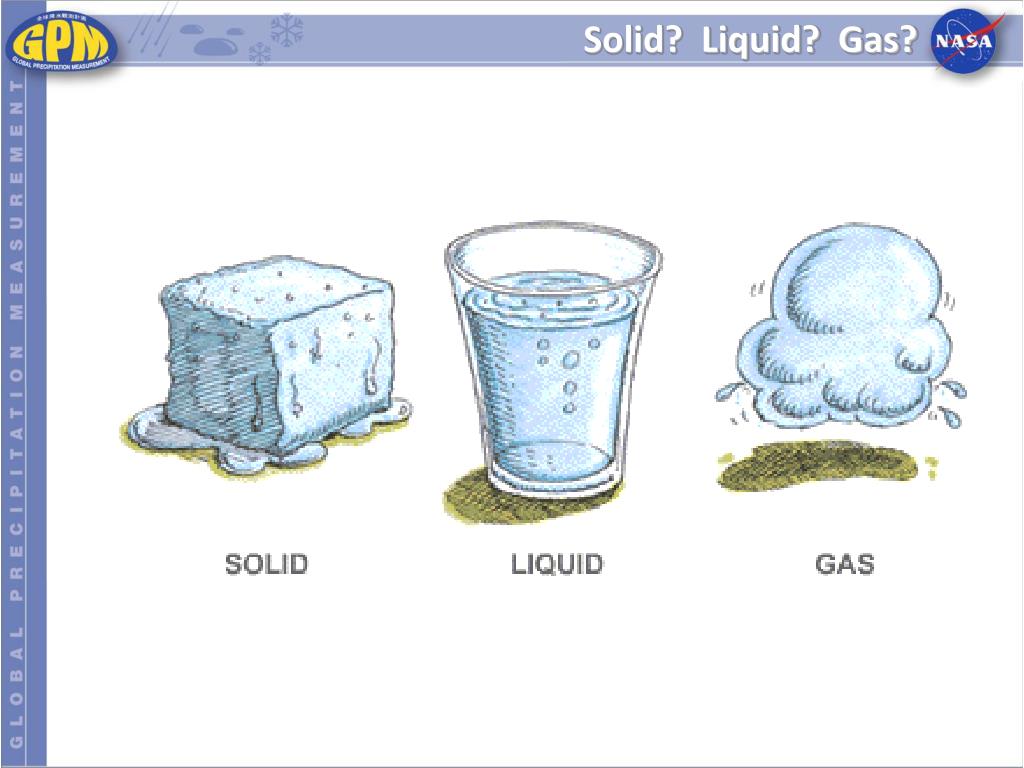
Solid Liquid Water . water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. Solid (ice), liquid (water), and gas (steam). In the solid state, water exists in the form of ice or. water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: Because water seems so ubiquitous,. water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. there are 3 different forms of water, or h 2 o: The water molecules are pushed.
from www.slideserve.com
Solid (ice), liquid (water), and gas (steam). Because water seems so ubiquitous,. In the solid state, water exists in the form of ice or. water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. The water molecules are pushed. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. there are 3 different forms of water, or h 2 o: the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense.
PPT Earth ’ s Water Cycle for Elementary School Students PowerPoint Presentation ID6211233
Solid Liquid Water Solid (ice), liquid (water), and gas (steam). Because water seems so ubiquitous,. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. Solid (ice), liquid (water), and gas (steam). there are 3 different forms of water, or h 2 o: In the solid state, water exists in the form of ice or. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. The water molecules are pushed. water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid.
From www.bbc.com
What is water? BBC Bitesize Solid Liquid Water the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: The water molecules are pushed. Because water seems so ubiquitous,. Solid (ice), liquid (water), and gas (steam). water, substance composed. Solid Liquid Water.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Illustration Solid Liquid Water water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds. Solid Liquid Water.
From www.wisegeek.com
What Are the Properties of Liquids? (with picture) Solid Liquid Water The water molecules are pushed. In the solid state, water exists in the form of ice or. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: there are 3. Solid Liquid Water.
From www.expii.com
Unique Densities (Water) — Properties & Examples Expii Solid Liquid Water there are 3 different forms of water, or h 2 o: The water molecules are pushed. Because water seems so ubiquitous,. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. the open structure of ice that allows for maximum hydrogen bonding explains. Solid Liquid Water.
From www.ase.org.uk
Solids, liquids and gases Solid Liquid Water Because water seems so ubiquitous,. there are 3 different forms of water, or h 2 o: because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. Solid (ice), liquid (water), and gas (steam). water’s lower density in its solid form is due to. Solid Liquid Water.
From earthhow.com
States of Water Gas, Liquid and Solid Earth How Solid Liquid Water water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. In the solid state, water exists in the form of. Solid Liquid Water.
From www.naturphilosophie.co.uk
Changing States Fundamental Phases of Matter NaturPhilosophie Solid Liquid Water Solid (ice), liquid (water), and gas (steam). The water molecules are pushed. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. there are 3 different. Solid Liquid Water.
From www.youtube.com
States of Matter Solid, Liquid, Gases. Interesting Animated Lesson For Children YouTube Solid Liquid Water water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. The water molecules are pushed. there are 3 different forms of water, or h 2 o: water, substance composed. Solid Liquid Water.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement and energy of the Solid Liquid Water water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen. Solid Liquid Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download ID2019622 Solid Liquid Water The water molecules are pushed. water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. Because water seems so ubiquitous,. there are 3 different forms of water, or h 2 o: the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. . Solid Liquid Water.
From www.sciencelearn.org.nz
Water solid, liquid and gas — Science Learning Hub Solid Liquid Water water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. In the solid state, water exists in the form of ice or. Because water seems so ubiquitous,. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules.. Solid Liquid Water.
From exofzvwyd.blob.core.windows.net
Is Water A Solid Liquid Or Gas at Ellen Jimenez blog Solid Liquid Water water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. there are 3 different forms of water, or h. Solid Liquid Water.
From circuitdbseriatim.z13.web.core.windows.net
Solid Liquid Gas Diagram Solid Liquid Water In the solid state, water exists in the form of ice or. there are 3 different forms of water, or h 2 o: Because water seems so ubiquitous,. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. water (h2o) is a polar inorganic compound that is at room. Solid Liquid Water.
From exotuafat.blob.core.windows.net
Liquid Vs Solid at James Foerster blog Solid Liquid Water The water molecules are pushed. Because water seems so ubiquitous,. Solid (ice), liquid (water), and gas (steam). water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. the open structure of. Solid Liquid Water.
From www.pinterest.com
The arrangement of water molecules in solid ice, liquid water and water vapour Changes in Solid Liquid Water water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. there are 3 different forms of water, or h 2 o: Because water seems so ubiquitous,. The water molecules are pushed.. Solid Liquid Water.
From mungfali.com
Matter Solids Liquids And Gases Solid Liquid Water The water molecules are pushed. water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: In the solid state, water exists in the form of ice or. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with. Solid Liquid Water.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid Liquid Water Because water seems so ubiquitous,. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. Solid (ice), liquid (water), and gas (steam). In the solid state,. Solid Liquid Water.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid Liquid Water the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. because of its polarity, a molecule of water in the liquid or solid state can. Solid Liquid Water.
From allaboutchemistry123.blogspot.com
What are solid, liquid, and gases? All About Chemistry Solid Liquid Water Because water seems so ubiquitous,. water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: The water molecules are pushed. there are 3 different forms of water, or h 2 o: In the solid state, water exists in the form of ice or. water (h2o) is a polar. Solid Liquid Water.
From www.expii.com
Density of Water — Comparison of Solid vs. Liquid Expii Solid Liquid Water water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. The water molecules are pushed. In the solid state, water exists in the form of ice or. water (h2o) is a. Solid Liquid Water.
From www.snexplores.org
Explainer What are the different states of matter? Solid Liquid Water Solid (ice), liquid (water), and gas (steam). In the solid state, water exists in the form of ice or. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. water’s lower density in its solid form is due to the way hydrogen bonds are. Solid Liquid Water.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Solid Liquid Water water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: Because water seems so ubiquitous,. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and. Solid Liquid Water.
From www.alamy.com
matter in different states for example water. solid, liquid, gas and plasma. molecular form Solid Liquid Water the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. water’s lower density in its solid form is due to the way hydrogen bonds are. Solid Liquid Water.
From www.vecteezy.com
Solid Liquid Gas Vector Art, Icons, and Graphics for Free Download Solid Liquid Water because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. the open structure of ice that allows for maximum. Solid Liquid Water.
From www.slideserve.com
PPT Earth ’ s Water Cycle for Elementary School Students PowerPoint Presentation ID6211233 Solid Liquid Water Because water seems so ubiquitous,. water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. The water molecules are pushed. Solid (ice), liquid (water), and gas (steam).. Solid Liquid Water.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid Liquid Water Because water seems so ubiquitous,. The water molecules are pushed. the open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense. water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: water (h2o) is a polar inorganic compound that is at. Solid Liquid Water.
From chemistry.stackexchange.com
phase Thermodynamics of SolidLiquid Chemistry Stack Exchange Solid Liquid Water because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. Because water seems so ubiquitous,. water’s lower density in its solid form is due to the. Solid Liquid Water.
From ar.inspiredpencil.com
Water Solid Solid Liquid Water water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. The water molecules are pushed. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. there are 3 different forms of water, or h 2 o:. Solid Liquid Water.
From vinsweb.org
States of Matter Vermont Institute of Natural Science Solid Liquid Water water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. there are 3 different forms of water, or h 2 o: In the solid state, water exists in the form of ice or. The water molecules are pushed. water, substance composed of the. Solid Liquid Water.
From schematicdatavenin77.z5.web.core.windows.net
Diagram Of Liquid Water Solid Liquid Water In the solid state, water exists in the form of ice or. there are 3 different forms of water, or h 2 o: Because water seems so ubiquitous,. water’s lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes: The water molecules are pushed. the open structure of ice. Solid Liquid Water.
From www.youtube.com
Science Three states of water, solid, liquid & gas Water change form drawing for kid Solid Liquid Water there are 3 different forms of water, or h 2 o: Because water seems so ubiquitous,. water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. Solid (ice), liquid (water), and gas (steam). water’s lower density in its solid form is due to. Solid Liquid Water.
From www.dreamstime.com
Water States of Matter Phase. Change of State for Water Diagram Stock Vector Illustration of Solid Liquid Water water (h2o) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an. water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. because of its polarity, a molecule of water in the liquid or solid state can form. Solid Liquid Water.
From www.techexplorist.com
Elements can be solid and liquid at the same time Solid Liquid Water water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. In the solid state, water exists in the form of ice or. water’s lower density in. Solid Liquid Water.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Solid Liquid Water water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. In the solid state, water exists in the form of ice or. Because water seems so ubiquitous,. there are 3 different forms of water, or h 2 o: the open structure of ice that allows for maximum hydrogen bonding explains. Solid Liquid Water.
From www.alamy.com
Density of matter with gas, liquid and solid water states outline diagram Stock Vector Image Solid Liquid Water water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid. there are 3 different forms of water, or h 2 o: Solid (ice), liquid (water), and gas (steam). The water molecules are pushed. because of its polarity, a molecule of water in the liquid or solid state can form up. Solid Liquid Water.