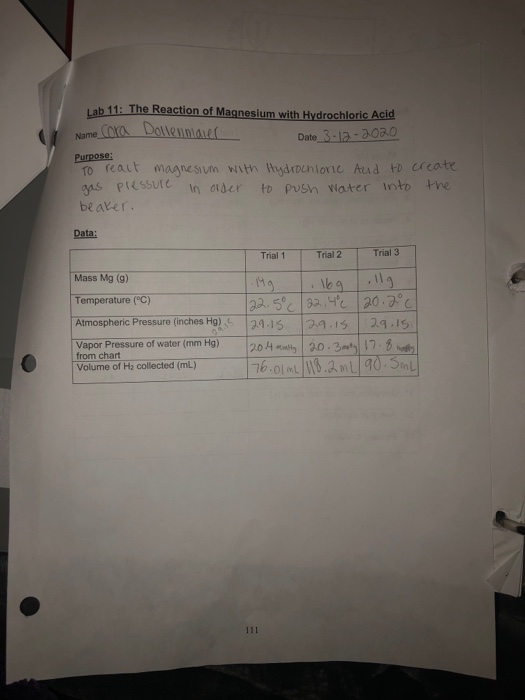
Magnesium And Hydrochloric Acid Experiment Lab Report . In this experiment you will determine the volume of the hydrogen gas that is produced when a sample. magnesium and dilute hydrochloric acid react together according to the equation below: the equation for the reaction is: Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Magnesium + hydrochloric acid →. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. in this experiment you will determine the volume of hydrogen gas that is produced when a sample of magnesium reacts with 6m hydrochloric acid. the reaction of magnesium with hydrochloric acid.
from www.chegg.com
Magnesium + hydrochloric acid →. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. In this experiment you will determine the volume of the hydrogen gas that is produced when a sample. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. magnesium and dilute hydrochloric acid react together according to the equation below: Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. in this experiment you will determine the volume of hydrogen gas that is produced when a sample of magnesium reacts with 6m hydrochloric acid.
Solved Lab 11 The Reaction of Magnesium with Hydrochloric
Magnesium And Hydrochloric Acid Experiment Lab Report Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. the reaction of magnesium with hydrochloric acid. In this experiment you will determine the volume of the hydrogen gas that is produced when a sample. in this experiment you will determine the volume of hydrogen gas that is produced when a sample of magnesium reacts with 6m hydrochloric acid. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. magnesium and dilute hydrochloric acid react together according to the equation below: the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. the equation for the reaction is: Magnesium + hydrochloric acid → magnesium chloride + hydrogen. by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. Magnesium + hydrochloric acid →.
From www.youtube.com
Magnesium Hydroxide and Hydrochloric Acid Reaction with Universal Magnesium And Hydrochloric Acid Experiment Lab Report carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. In this experiment you will determine the volume of the hydrogen gas that is produced when a sample. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and. Magnesium And Hydrochloric Acid Experiment Lab Report.
From edu.rsc.org
The rate of reaction of magnesium with hydrochloric acid Experiment Magnesium And Hydrochloric Acid Experiment Lab Report In this experiment you will determine the volume of the hydrogen gas that is produced when a sample. the reaction of magnesium with hydrochloric acid. Magnesium + hydrochloric acid →. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. Mg(s) + 2hcl(aq) → mgcl 2. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.youtube.com
Magnesium & Hydrochloric Acid Reaction YouTube Magnesium And Hydrochloric Acid Experiment Lab Report the equation for the reaction is: by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. Magnesium + hydrochloric acid →. the purpose of this experiment was to determine how. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.youtube.com
What Happens when Magnesium reacts with Acetic Acid And Dilute Magnesium And Hydrochloric Acid Experiment Lab Report the reaction of magnesium with hydrochloric acid. this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. by inducing a. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.youtube.com
Magnesium Ribbon and Hydrochloric Acid Experiment YouTube Magnesium And Hydrochloric Acid Experiment Lab Report Magnesium + hydrochloric acid →. the reaction of magnesium with hydrochloric acid. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.academia.edu
(PDF) Magnesium and HCl Lab Report Ann YIP Academia.edu Magnesium And Hydrochloric Acid Experiment Lab Report this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. Magnesium + hydrochloric acid →. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. In this experiment you will determine the volume. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.markedbyteachers.com
Investigating the rate of reaction between magnesium and hydrochloric Magnesium And Hydrochloric Acid Experiment Lab Report In this experiment you will determine the volume of the hydrogen gas that is produced when a sample. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. the purpose of this experiment was to determine how much. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.markedbyteachers.com
Finding the effect of concentration on the rate of reaction of Magnesium And Hydrochloric Acid Experiment Lab Report magnesium and dilute hydrochloric acid react together according to the equation below: this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid,. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.scribd.com
Magnesium and Hydrochloric Acid Lab Report PDF Gases Mole (Unit) Magnesium And Hydrochloric Acid Experiment Lab Report the equation for the reaction is: by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. in this experiment. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.studypool.com
SOLUTION Investigation experiment to show the reaction between Magnesium And Hydrochloric Acid Experiment Lab Report the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. in this experiment you will determine the volume of hydrogen gas. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.scribd.com
Experiment 4 Applied PDF Magnesium Hydrochloric Acid Magnesium And Hydrochloric Acid Experiment Lab Report in this experiment you will determine the volume of hydrogen gas that is produced when a sample of magnesium reacts with 6m hydrochloric acid. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. In this. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.scribd.com
Magnesium Reacts With Dilute Hydrochloric Acid in a Conical Flask Which Magnesium And Hydrochloric Acid Experiment Lab Report this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. the reaction of magnesium with hydrochloric acid. by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. carrying. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.researchgate.net
Reaction of magnesium with HCl. Observation and analysis 1. You have Magnesium And Hydrochloric Acid Experiment Lab Report the reaction of magnesium with hydrochloric acid. the equation for the reaction is: Magnesium + hydrochloric acid →. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. in this experiment you will determine the volume of hydrogen gas that is produced when a sample. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.scribd.com
Hydrochloric Acid and Magnesium Lab PDF Reaction Rate Chemical Magnesium And Hydrochloric Acid Experiment Lab Report magnesium and dilute hydrochloric acid react together according to the equation below: the equation for the reaction is: Magnesium + hydrochloric acid →. by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium. Magnesium And Hydrochloric Acid Experiment Lab Report.
From segudanggambar798.blogspot.com
1. Solid Magnesium Oxide Is Reacted With Hydrochloric Acid Single Magnesium And Hydrochloric Acid Experiment Lab Report in this experiment you will determine the volume of hydrogen gas that is produced when a sample of magnesium reacts with 6m hydrochloric acid. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. the reaction of magnesium with hydrochloric acid. carrying out a. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.slideserve.com
PPT IB Chemistry PowerPoint Presentation, free download ID2738432 Magnesium And Hydrochloric Acid Experiment Lab Report the reaction of magnesium with hydrochloric acid. Magnesium + hydrochloric acid →. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. the equation for the reaction is: magnesium and dilute hydrochloric acid react together according to the equation below: In this experiment you. Magnesium And Hydrochloric Acid Experiment Lab Report.
From myans.bhantedhammika.net
Molar Volume Of A Gas Lab Magnesium + Hydrochloric Acid Magnesium And Hydrochloric Acid Experiment Lab Report Magnesium + hydrochloric acid → magnesium chloride + hydrogen. In this experiment you will determine the volume of the hydrogen gas that is produced when a sample. Magnesium + hydrochloric acid →. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.scribd.com
Magnesium and Hydrochloric Acid Reaction Lab PDF Chemical Reactions Magnesium And Hydrochloric Acid Experiment Lab Report carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. Magnesium + hydrochloric acid →. magnesium and dilute hydrochloric acid react together according to the equation below: by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. the reaction of. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.numerade.com
SOLVEDREPORT SUMMARY value of R (2pts) Questions (Ipts) The hydrogen Magnesium And Hydrochloric Acid Experiment Lab Report Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. Magnesium + hydrochloric acid →. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of.. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.youtube.com
Magnesium and Hydrochloric Acid Lab YouTube Magnesium And Hydrochloric Acid Experiment Lab Report the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. in this experiment you will determine the volume of hydrogen gas that is produced when a sample of magnesium reacts with 6m hydrochloric acid. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. the equation. Magnesium And Hydrochloric Acid Experiment Lab Report.
From childhealthpolicy.vumc.org
😝 Rate of reaction experiment magnesium ribbon and hydrochloric acid Magnesium And Hydrochloric Acid Experiment Lab Report magnesium and dilute hydrochloric acid react together according to the equation below: by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10. Magnesium And Hydrochloric Acid Experiment Lab Report.
From studylib.net
The Reaction of Magnesium with Hydrochloric Acid Lab Magnesium And Hydrochloric Acid Experiment Lab Report magnesium and dilute hydrochloric acid react together according to the equation below: by inducing a chemical reaction by reacting a strip of magnesium to hydrochloric acid, the. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. carrying out a laboratory experiment where the. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.studocu.com
Lab 07 Reaction of Magnesium with Hydrochloric Acid (H2 Gen)1 The Magnesium And Hydrochloric Acid Experiment Lab Report in this experiment you will determine the volume of hydrogen gas that is produced when a sample of magnesium reacts with 6m hydrochloric acid. the equation for the reaction is: the reaction of magnesium with hydrochloric acid. Magnesium + hydrochloric acid →. magnesium and dilute hydrochloric acid react together according to the equation below: In this. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.studocu.com
Summative Science Lab Report Science Lab Report Standardisation of a Magnesium And Hydrochloric Acid Experiment Lab Report Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. the reaction of magnesium with hydrochloric acid. the purpose of this experiment was to determine how. Magnesium And Hydrochloric Acid Experiment Lab Report.
From vdocuments.mx
1 Magnesium reacts with dilute hydrochloric acid, HCl [PDF Document] Magnesium And Hydrochloric Acid Experiment Lab Report carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. In this experiment you will determine the volume of the hydrogen gas that is produced when a sample. the equation for the reaction is: Magnesium + hydrochloric acid →. the purpose of this experiment was to. Magnesium And Hydrochloric Acid Experiment Lab Report.
From webapi.bu.edu
😱 Rate of reaction experiment magnesium ribbon and hydrochloric acid Magnesium And Hydrochloric Acid Experiment Lab Report the reaction of magnesium with hydrochloric acid. the equation for the reaction is: the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of.. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.studypool.com
SOLUTION Scientific investigation how temperature affects the rate of Magnesium And Hydrochloric Acid Experiment Lab Report Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. in this experiment you will determine the volume of hydrogen gas that is produced when a sample of magnesium reacts with 6m hydrochloric acid. this. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.chegg.com
Solved Lab 11 The Reaction of Magnesium with Hydrochloric Magnesium And Hydrochloric Acid Experiment Lab Report this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students. Magnesium And Hydrochloric Acid Experiment Lab Report.
From complianceportal.american.edu
💋 Rate of reaction of magnesium with hydrochloric acid. Rate of Magnesium And Hydrochloric Acid Experiment Lab Report Magnesium + hydrochloric acid →. the equation for the reaction is: the reaction of magnesium with hydrochloric acid. this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. the purpose of this experiment. Magnesium And Hydrochloric Acid Experiment Lab Report.
From cityraven.com
😂 Rate of reaction of magnesium and hydrochloric acid. Concentration Of Magnesium And Hydrochloric Acid Experiment Lab Report Magnesium + hydrochloric acid →. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. this experiment will be an opportunity for you to design an apparatus to collect hydrogen gas formed in the reaction between magnesium metal and. In this experiment you will determine the. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.markedbyteachers.com
Investigate the rate of reaction with magnesium and different Magnesium And Hydrochloric Acid Experiment Lab Report the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. Magnesium + hydrochloric acid →. the reaction of magnesium with hydrochloric acid. the. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.chegg.com
Solved Lab 11 The Reaction of Magnesium with Hydrochloric Magnesium And Hydrochloric Acid Experiment Lab Report in this experiment you will determine the volume of hydrogen gas that is produced when a sample of magnesium reacts with 6m hydrochloric acid. the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. magnesium and dilute hydrochloric acid react together according to the equation. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.sciencephoto.com
Magnesium reacts with hydrochloric acid Stock Image C028/0732 Magnesium And Hydrochloric Acid Experiment Lab Report the reaction of magnesium with hydrochloric acid. the equation for the reaction is: In this experiment you will determine the volume of the hydrogen gas that is produced when a sample. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between. Magnesium And Hydrochloric Acid Experiment Lab Report.
From studymoose.com
Lab Report Magnesium and Hydrochloric Acid Reaction StudyMoose Magnesium And Hydrochloric Acid Experiment Lab Report the purpose of this experiment was to determine how much hydrogen gas would be produced when a sample of magnesium metal reacts. the reaction of magnesium with hydrochloric acid. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced. Magnesium And Hydrochloric Acid Experiment Lab Report.
From www.markedbyteachers.com
How dose temperature affect the rate of reaction between magnesium and Magnesium And Hydrochloric Acid Experiment Lab Report Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g) students follow the rate of reaction between magnesium and the acid, by measuring the amount of gas produced at 10 second intervals. carrying out a laboratory experiment where the results are memorable either by their visual nature or by their tying together of. the reaction of magnesium. Magnesium And Hydrochloric Acid Experiment Lab Report.