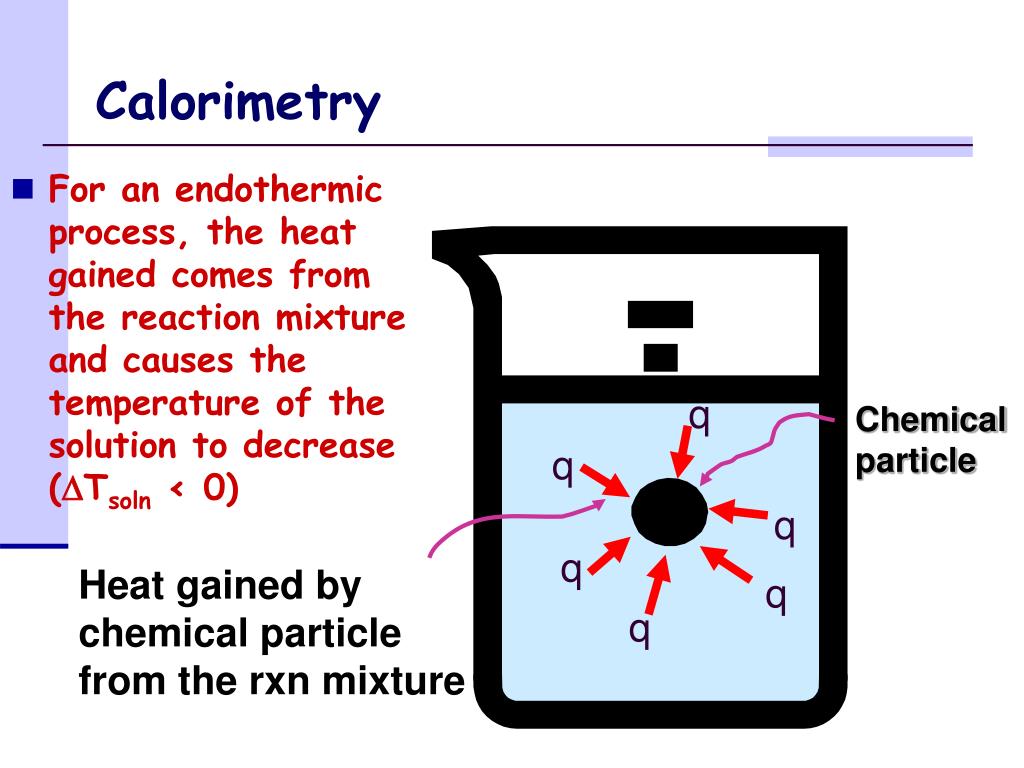
How To Calculate Q Of Calorimeter . One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the equilibrium temperature. The calibration is accomplished using a reaction with a known q, such. The calorimetry calculator can help you solve complex calorimetry problems. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The total heat energy released in the chemical reaction is: How to find the heat capacity of calorimeter? Then use equation \(\pageindex{2}\) to determine the heat capacity of the. Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. Total heat energy = q = \delta {q_.
from www.slideserve.com
The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. Then use equation \(\pageindex{2}\) to determine the heat capacity of the. It can analyze the heat exchange between up to 3 objects. The total heat energy released in the chemical reaction is: Total heat energy = q = \delta {q_. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. How to find the heat capacity of calorimeter? The calibration is accomplished using a reaction with a known q, such.
PPT Calorimetry PowerPoint Presentation, free download ID6133898
How To Calculate Q Of Calorimeter Total heat energy = q = \delta {q_. One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the equilibrium temperature. The total heat energy released in the chemical reaction is: Total heat energy = q = \delta {q_. It can analyze the heat exchange between up to 3 objects. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. How to find the heat capacity of calorimeter? Then use equation \(\pageindex{2}\) to determine the heat capacity of the. Additionally, it can find the. The calorimetry calculator can help you solve complex calorimetry problems. The calibration is accomplished using a reaction with a known q, such. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube How To Calculate Q Of Calorimeter Then use equation \(\pageindex{2}\) to determine the heat capacity of the. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. How to find the heat capacity of calorimeter? Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. One way to calibrate a. How To Calculate Q Of Calorimeter.
From courses.lumenlearning.com
9.2 Calorimetry General College Chemistry I How To Calculate Q Of Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The calibration is accomplished using a reaction with a known q, such. The total heat energy released in the chemical reaction is: Then use equation \(\pageindex{2}\) to determine the heat capacity of the. The heat capacity (c) of a body of matter. How To Calculate Q Of Calorimeter.
From duntanexhurrard.blogspot.com
DuntanexHurrard How To Calculate Q Of Calorimeter Additionally, it can find the. The calibration is accomplished using a reaction with a known q, such. It can analyze the heat exchange between up to 3 objects. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. The calorimetry calculator. How To Calculate Q Of Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 How To Calculate Q Of Calorimeter It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. The calorimetry calculator can help you solve complex calorimetry problems. The calibration is accomplished using a reaction with a known q, such. One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the equilibrium. How To Calculate Q Of Calorimeter.
From www.chegg.com
Solved How to calculate the calorimeter constant How To Calculate Q Of Calorimeter The total heat energy released in the chemical reaction is: It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure. How To Calculate Q Of Calorimeter.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper How To Calculate Q Of Calorimeter How to find the heat capacity of calorimeter? The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. The total heat energy released in the. How To Calculate Q Of Calorimeter.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube How To Calculate Q Of Calorimeter How to find the heat capacity of calorimeter? Total heat energy = q = \delta {q_. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. The calorimetry calculator can help you solve complex calorimetry problems. Bomb calorimeters require calibration to. How To Calculate Q Of Calorimeter.
From gioxognua.blob.core.windows.net
Calorimeter Constant In Chemistry at Zula Grant blog How To Calculate Q Of Calorimeter The calibration is accomplished using a reaction with a known q, such. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. How. How To Calculate Q Of Calorimeter.
From www.youtube.com
050 Calorimetry YouTube How To Calculate Q Of Calorimeter How to find the heat capacity of calorimeter? Then use equation \(\pageindex{2}\) to determine the heat capacity of the. The calorimetry calculator can help you solve complex calorimetry problems. The calibration is accomplished using a reaction with a known q, such. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. The heat capacity. How To Calculate Q Of Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation How To Calculate Q Of Calorimeter Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. How To Calculate Q Of Calorimeter.
From saylordotorg.github.io
Calorimetry How To Calculate Q Of Calorimeter Total heat energy = q = \delta {q_. One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the equilibrium temperature. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Additionally, it can find the. The calorimetry calculator can help you solve complex. How To Calculate Q Of Calorimeter.
From lessonlangdonprowl.z21.web.core.windows.net
Coffee Cup Calorimetry Calculator How To Calculate Q Of Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the equilibrium temperature. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Calculate heat, temperature change, and specific heat after thermal equilibrium is. How To Calculate Q Of Calorimeter.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo How To Calculate Q Of Calorimeter The total heat energy released in the chemical reaction is: How to find the heat capacity of calorimeter? The calorimetry calculator can help you solve complex calorimetry problems. The calibration is accomplished using a reaction with a known q, such. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when. How To Calculate Q Of Calorimeter.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to How To Calculate Q Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. The total heat energy released in the chemical reaction is: Additionally, it can find the. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid. How To Calculate Q Of Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry I How To Calculate Q Of Calorimeter It can analyze the heat exchange between up to 3 objects. Then use equation \(\pageindex{2}\) to determine the heat capacity of the. The calibration is accomplished using a reaction with a known q, such. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Total heat energy = q = \delta {q_.. How To Calculate Q Of Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How To Calculate Q Of Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the. How To Calculate Q Of Calorimeter.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry How To Calculate Q Of Calorimeter Total heat energy = q = \delta {q_. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. One way to calibrate a calorimeter is by mixing two quantities of water in it at. How To Calculate Q Of Calorimeter.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube How To Calculate Q Of Calorimeter It can analyze the heat exchange between up to 3 objects. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. How to find the heat capacity of calorimeter? The calibration is accomplished using a reaction with a known q, such. Bomb calorimeters require calibration to determine the heat capacity. How To Calculate Q Of Calorimeter.
From dxodvsdxa.blob.core.windows.net
Calorimeter In Physics Meaning at Dennis Flores blog How To Calculate Q Of Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. Then use equation \(\pageindex{2}\) to determine the heat capacity of the. How to find the heat capacity of calorimeter? It can. How To Calculate Q Of Calorimeter.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero How To Calculate Q Of Calorimeter Additionally, it can find the. One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the equilibrium temperature. The calibration is accomplished using a reaction with a known q, such. How to find the heat capacity of calorimeter? Calculate heat, temperature change, and specific heat after thermal equilibrium is reached. How To Calculate Q Of Calorimeter.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube How To Calculate Q Of Calorimeter Then use equation \(\pageindex{2}\) to determine the heat capacity of the. Total heat energy = q = \delta {q_. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. Calculate the value of q rxn for. How To Calculate Q Of Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube How To Calculate Q Of Calorimeter Additionally, it can find the. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. How to find the heat capacity of calorimeter? The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up. How To Calculate Q Of Calorimeter.
From en.ppt-online.org
What is enthalpy? online presentation How To Calculate Q Of Calorimeter Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. The calorimetry calculator can help you solve complex calorimetry problems. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. One way to calibrate a calorimeter is by mixing two quantities of. How To Calculate Q Of Calorimeter.
From giovdathz.blob.core.windows.net
How To Calculate The Heat Capacity Of Calorimeter at Brian Elizondo blog How To Calculate Q Of Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The calibration is accomplished using a reaction with a known q, such. The total heat energy released in the chemical reaction is: Total heat energy = q = \delta {q_. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter. How To Calculate Q Of Calorimeter.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo How To Calculate Q Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. One way to calibrate a calorimeter is by mixing two quantities of water. How To Calculate Q Of Calorimeter.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 How To Calculate Q Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. It can analyze the heat exchange between up to 3 objects. The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. The total heat energy released. How To Calculate Q Of Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 How To Calculate Q Of Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. Then use equation \(\pageindex{2}\) to. How To Calculate Q Of Calorimeter.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry How To Calculate Q Of Calorimeter The calibration is accomplished using a reaction with a known q, such. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. It can analyze the heat exchange between up to 3 objects. Then use equation \(\pageindex{2}\) to determine the heat. How To Calculate Q Of Calorimeter.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo How To Calculate Q Of Calorimeter It can analyze the heat exchange between up to 3 objects. The total heat energy released in the chemical reaction is: The calibration is accomplished using a reaction with a known q, such. Additionally, it can find the. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Total heat energy =. How To Calculate Q Of Calorimeter.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube How To Calculate Q Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree. The calorimetry calculator can help you solve complex calorimetry problems. One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the. How To Calculate Q Of Calorimeter.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 How To Calculate Q Of Calorimeter Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The calorimetry calculator can help you solve complex calorimetry problems. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. One way to calibrate a calorimeter is by mixing two quantities of. How To Calculate Q Of Calorimeter.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The How To Calculate Q Of Calorimeter The total heat energy released in the chemical reaction is: One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the equilibrium temperature. How to find the heat capacity of calorimeter? Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. It can analyze. How To Calculate Q Of Calorimeter.
From users.highland.edu
Calorimetry How To Calculate Q Of Calorimeter How to find the heat capacity of calorimeter? Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. One way to calibrate a calorimeter is by mixing two quantities of water in it at different temperatures and recording the equilibrium temperature. The calorimetry calculator can help you solve complex calorimetry problems. The. How To Calculate Q Of Calorimeter.
From www.youtube.com
Calorimetry calculation YouTube How To Calculate Q Of Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. The total heat energy released in the chemical reaction is: The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when. How To Calculate Q Of Calorimeter.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool How To Calculate Q Of Calorimeter How to find the heat capacity of calorimeter? The total heat energy released in the chemical reaction is: Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Bomb calorimeters require calibration to determine the heat capacity of the calorimeter and ensure accurate results. The heat capacity (c) of a. How To Calculate Q Of Calorimeter.